Extra Credit 44
- Page ID
- 83016
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.3
If a sample of iron and a sample of zinc come into contact, the zinc corrodes but the iron does not. If a sample of iron comes into contact with a sample of copper, the iron corrodes but the copper does not. Explain this phenomenon.
S17.6.3
Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. Corrosion is the process of returning a metal to its natural state as an ore, and is a form of oxidation. Sacrificial anodes protect the cathode. In order to answer this question, we look at the standard reduction potentials (SRP), which indicates how likely it is for a certain metal to be reduced. A metal with a higher SRP is more likely to be reduced, and a metal with a more negative or smaller SRP is more likely to be oxidized.
Situation 1: A sample of iron and a sample of zinc come into contact. The zinc corrodes but the iron does not. We will look at the chart of standard reduction potentials to see which metal is more likely to be reduced and which metal is more likely to be oxidized. We find that iron has an SRP of -0.44 V, while zinc has an SRP of -0.76 V. Iron has a higher SRP than zinc, so therefore it is more likely to be reduced and less likely to be oxidized. This means that the zinc, with the lower SRP is more likely to get oxidized. Since corrosion is a form of oxidation, we can conclude that the zinc will corrode but the iron will not. In this case, zinc acts as the sacrificial anode.
Situation 2: A sample of iron and a sample of copper come into contact. The iron corrodes but the copper does not. Again, we will look at the activity series and find that the SRP of iron is -0.44 V while the SRP of copper is 0.34 V. Notice that copper has a higher SRP than iron, so therefore it is more likely to be reduced. Iron has a lower SRP, and is more likely to be oxidized. This means that iron is the sacrificial anode. Iron will corrode, but the copper will not.
Here is a link to the table of Standard Reduction Potentials:
Q12.5.16
Use the PhET Reactions & Rates interactive simulation to simulate a system. On the “Single collision” tab of the simulation applet, enable the “Energy view” by clicking the “+” icon. Select the first A+BC⟶AB+C reaction (A is yellow, B is purple, and C is navy blue). Using the “straight shot” default option, try launching the A atom with varying amounts of energy. What changes when the Total Energy line at launch is below the transition state of the Potential Energy line? Why? What happens when it is above the transition state? Why?
S12.5.16
Activation Energy : the minimum quantity of energy that the reacting species must possess in order to undergo a specified reaction.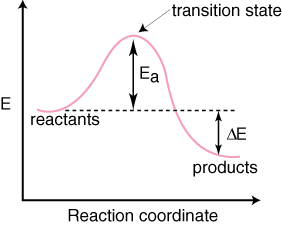
Transition State : the state corresponding to the highest potential energy along this reaction coordinate.
When the Total Energy line at launch is below the transition state of the Potential Energy line, then the reaction does not go through completion. When it is launched with such little energy, the A particle does not hit the BC particle with enough energy for the reaction to proceed. Therefore the overall reaction cannot carry on with such little initial energy. When the Total Energy line is launched above the transition state, A is launched with enough energy to overcome the activation energy, and the reaction can proceed.
The diagram to the right shows the graph of a chemical reaction progression. In order for the reaction to proceed, there needs to be enough energy to overcome the activation energy.
Q12.3.7
Radioactive phosphorus is used in the study of biochemical reaction mechanisms because phosphorus atoms are components of many biochemical molecules. The location of the phosphorus (and the location of the molecule it is bound in) can be detected from the electrons (beta particles) it produces:
\[_{15}^{32}P⟶^{32}_{16}S+e^{−1}\]
\[Rate = 4.85×10^{-2} day^{−1}[^{32}P]\]
What is the instantaneous rate of production of electrons in a sample with a phosphorus concentration of 0.0033 M?
S12.3.7
In this problem the rate is given in terms of a rate constant and the concentration of a reactant. Since the units of the rate constant \(k\) are \(day^{−1}\), the reaction is first order. This applies to any unit of time. For example, if the units of \(k\) are \(sec^{-1}\) the reaction would be first order. This is because the rate is given in concentration per unit of time.
Now, plug in the given concentration of phosphorus .0033M into the given rate equation to get the instantaneous rate of production of electrons in the sample:
\[Rate = 4.85×10^{-2} day^{−1}[^{32}P]\]
\[Rate = 4.85×10^{-2} day^{−1}[.0033M] = 1.60x10{-4} \scriptsize\frac{M}{day}\]
Q21.4.11
Write a nuclear reaction for each step in the formation of \(_{84}^{218}Po\) from \(_{92}^{238}U\) , which proceeds by a series of decay reactions involving the step-wise emission of α, β, β, α, α, α, α particles, in that order.
S21.4.11
An alpha particle, α, a positively charged particle consisting of two protons and two neutrons, written as \(_{2}^{4}He\).
Emission by Alpha particles :
- Emitting a alpha particle decreases neutron number by two
- Emitting a alpha particle decreases proton number by two
- Emitting a alpha particle decreases mass number by four
A beta particle β, can either be a positron or an electron. Since the question does not specify, we assume that it is an electron. A beta particle's mass is negligible compared to the mass of a proton or neutron and has a charge of -1. It can be written as \( _{-1}^{0}β\).
Emission by beta particles (negative time = electron) :
- Emitting a negative beta particle decreases neutron number by one
- Emitting a negative beta particle increases proton number by one
- Emitting a negative beta particle does not change mass number
Remember that "nuclear nomenclature" can be written as: \(_{Z}^{A}X\) where
A: Number of nucleons (mass number) Z: The number of protons (atomic number) X: The particle or Element
In the first reaction, an alpha particle is emitted : \(_{92}^{238}U⟶_{90}^{234}Th+_2^4He\).
From above, we know that emitting an alpha particle decreases the proton number by two, and decreases the mass number by four. For the reactants side, Z=92 and A=238. The protons decrease by 2, so 92-2=90. There are now 90 protons, which corresponds to the element Thorium. Now look at mass number, A. We know emitting an alpha particle decreases the mass number by four. 238-4=234. This is the mass number that goes with Thorium.
In the second reaction, a beta particle is emitted : \(_{90}^{234}Th⟶_{91}^{234}Pa+_{-1}^0β\)
From above, we know that emitting a beta particle increases the proton number by one, and does not affect the mass number. For the reactants side, Z = 90 and A = 234. Since emitting a beta particle does not change the mass number, it stays at 234. We know that Z must increase by 1. 90 + 1 = 91. Look at the periodic table to find which element has the atomic number 91.
In the third reaction, a beta particle is emitted : \(_{91}^{234}Pa⟶_{92}^{234}U+_{-1}^0β\)
In the fourth reaction, an alpha particle is emitted : \(_{92}^{234}U⟶_{90}^{230}Th+_2^4He\)
In the fifth reaction, an alpha particle is emitted : \(_{90}^{230}Th⟶_{88}^{226}Ra+_2^4He\)
In the sixth reaction, an alpha particle is emitted : \(_{88}^{226}Ra⟶_{86}^{222}Rn+_2^4He\)
In the seventh reaction, an alpha particle is emitted : \(_{86}^{222}Ra⟶_{84}^{218}Po+_2^4He\)
For each step, follow the same instructions for step one and step two. The mass of the products should equal the mass of the reactants, and the number of protons in the reactants should equal the number of protons in the products. To verify that these reactions are balanced correctly, check that:
- Sum of A of reactants = Sum of A of products
- Sum of Z of reactants = Sum of Z of products
- Make sure that when the number of protons changes (Z), the corresponding element is used.
Q20.2.15
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
a. \(Pt^{2+}{}_{(aq)} + Ag_{(s)} →\)
b. \(HCN_{(aq)} + NaOH_{(aq)}→\)
c. \(Fe(NO_{3})_{3(aq)} + NaOH_{(aq)}→\)
d. \(CH_{4(g)} + O_{2(g)}→\)
S20.2.15
In order to solve this, we must first know the difference between the listed reactions and what to look for.
- Acid base reaction: a type of chemical process typified by the exchange of one or more hydrogen ions, \(H^{+}\). The neutralization of an acid-base reaction results in the formation of water.
- Precipitation reaction : Precipitation reactions transform ions into an insoluble salt in aqueous solution.
- Redox reaction : An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species
a. \(Pt^{2+}{}_{(aq)} + Ag_{(s)} →\) : When a metal in elemental form is placed in a solution of another metal salt it may be more energetically feasible for this "elemental metal" to exist as an ion and the "ionic metal" to exist as the element. Therefore the elemental metal will "displace" the ionic metal and the two swap places. When we look at the Activity Series of metals, we see that the elemental metal, Ag, is higher on the activity series, therefore will displace the Pt ion.
\(Pt^{2+}{}_{(aq)} + Ag_{(s)} → Ag^{2+}{}_{(aq)} + Pt_{(s)} \). Electrons were transferred; therefore, this is a redox reaction.
b. \(HCN_{(aq)} + NaOH_{(aq)}→ NaHCN_{(aq)} + H_{2}O_{(l)}\) : This is an acid-base reaction. In an acid-base neutralization, an acid and a base react to form water and salt. In order for the reaction to carry out, there must be a transfer of protons between acids and bases. Proton acceptors and proton donors are the basis for these reactions and are also referred to as conjugate bases and acids. Here, we see that protons are transferred between the acids and bases.
c. \(Fe(NO_{3})_{3(aq)} + NaOH_{(aq)}→Fe(OH)_{3{(s)}} + 3NaNO_{3{(l)}}\) : This is a precipitate reaction. This reaction follows the pattern: AB + CD → CB + AD, which is a double replacement reaction. This reaction forms a precipitate because Fe(OH) is not soluble therefore forms a solid precipitate.
Solubility rules can be found here : https://chem.libretexts.org/Core/Phy...lubility_Rules
d. \(CH_{4(g)} + O_{2(g)}→CO_{2(g)} + 2H_{2{(g)}}\) : This a redox reaction. An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. To show this is a redox reaction, we must look at the oxidation states of each element before and after the reaction.
1) \(CH_{4(g)}\) : C has an oxidation state of -4 and H has an oxidation state of +1
2) \(O_{2(g)}\) : O has an oxidation state of 0.
3) \(CO_{2(g)}\) : C has an oxidation state of +4 and O has an oxidation state of -2.
4) \(2H_{2{(g)}}\): H has an oxidation state of 0.
From these values, we can see that there clearly is a change in oxidation states, therefore electrons were transferred.
Oxidation rules can be found here : https://chem.libretexts.org/Core/Ana...xidation_State
Q20.5.10
Describe how an electrochemical cell can be used to measure the solubility of a sparingly soluble salt.
S20.5.10
To help with this problem, we will first understand what an electrochemical cell is. An electrochemical cell is an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. These cells contain a cathode and an anode. A cathode is where reduction takes place, and an anode is where oxidation takes place. Each cell also has electrodes, which provide an electrical connection between the anode and cathode.
In order to do this, we must use the Nernst Equation. The Nernst Equation enables the determination of cell potential under non-standard conditions. It relates the measured cell potential to the reaction quotient and allows the accurate determination of equilibrium constants (including solubility constants).
The Nernst Equation under equilibrium conditions is
\[E^{o}_{cell}=\dfrac{RT}{nF}\ln K\]
- \(E^{o}_{cell}\) : Standard Electrode Potential (Cathode-Anode)
- R: Universal Gas Constant (make sure units match)
- T: Temperature (in Kelvin)
- n: # of electrons transferred. Found from a balanced redox reaction.
- F: Faraday's constant
- K: Equilibrium Constant. In this case it is the solubility, typically \(\frac{[products]}{[reactants]}\)
Using this equation, we can isolate the \(K\) part of the equation and find the concentration, or solubility.
Q24.6.6
Do strong-field ligands favor a tetrahedral or a square planar structure? Why?
S24.6.6
In order to answer this questions, we will define a few things first to help understand how orbitals are filled.
Aufbau principle: Electrons are going to occupy the orbitals in the order that minimizes the overall energy of the atom or molecule (Build up)
Hund’s rule: Electrons will fill orbitals of identical energy without pairing first (It costs energy to pair electrons).
P: Spin pairing energy. Spin pairing energy refers to the energy associated with paired electrons sharing one orbital and its effect on the molecules surrounding it.
Below are the crystal field splitting diagrams. Strong field ligands have a greater \(∆_o\). According to the Aufbau principle, electrons will occupy the orbitals in the order that minimizes the overall energy of the atom or molecule. For weak field ligands, \(∆_o\) < P, so the electrons will favor a high spin configuration. Conversely, strong field ligands have \(∆_o\) > P, so the electrons will have a low spin configuration. Whether a complex is high spin or low spin depends on two main factors: the crystal field splitting energy and the pairing energy. The electrons will take the path of least resistance--the path that requires the least amount of energy. If the paring energy is greater than Δ, then electrons will move to a higher energy orbital because it takes less energy. If the pairing energy is less than Δ, then the electrons will pair up rather than moving singly to a higher energy orbital.
In a tetrahedral complex, Δt is relatively small even with strong-field ligands as there are fewer ligands to bond with. It is rare for the Δt of tetrahedral complexes to exceed the pairing energy. Usually, electrons will move up to the higher energy orbitals rather than pair. Because of this, most tetrahedral complexes are high spin.
In square planar complexes Δ will almost always be large, even with a weak-field ligand. Electrons tend to be paired rather than unpaired because paring energy is usually much less than Δ. Therefore, square planar complexes are usually low spin.
Therefore, strong field ligands favor a square planar structure.
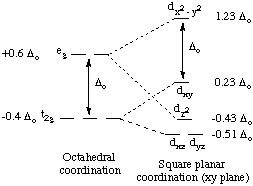
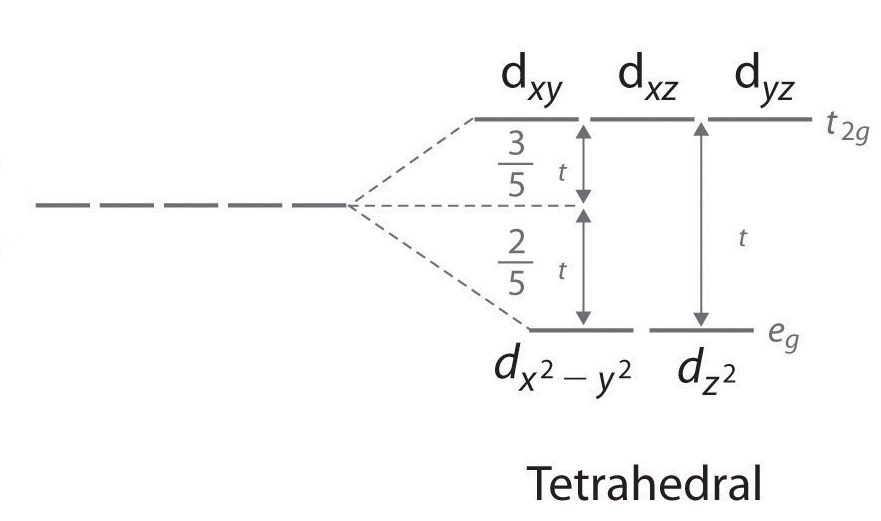
Q14.7.11
A particular reaction was found to proceed via the following mechanism:
- A + B → C + D (slow)
- 2C → E (fast)
- E + A → B + F (fast)
What is the overall reaction? Is this reaction catalytic, and if so, what species is the catalyst? Identify the intermediates.
S14.7.11
1) In order to find the overall reaction, we must add up each step wise reaction. To do this, add all the reactants and add all the products we get:
\(A + B + 2C + E + A ⟶ C + E + D + B + F\)
Combine like terms of the same species : \(2A + B + 2C + E → C + D + E + B + F\)
Finally, if a species appear on both sides, cancel them out. In this reaction, B and E appear both on the products side once and on the reacts side once. C appears once on the products side and twice on the reactants side, so that leaves 1 C on the reactants side.
\(A + \require{cancel} \cancel{B} + \cancel{2C} + \cancel{E} + A ⟶ \cancel{C} + \cancel{E} + D + \cancel{B} + F\)
Overall reaction : \(2A + C ⟶ D + F\)
2) This reaction is catalytic. A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. B is a catalyst because it speeds up the reaction and is unchanged during the net reaction.
3) A reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) and reacts further to give the directly observed products of a chemical reaction. An intermediate is produced and then consumed. If we look at step 1 of the solution, we see that both C and E are produced and then consumed in a later step. However, an intermediate does not appear in the overall reaction. Therefore the intermediate is E.

