Extra Credit 29
- Page ID
- 82999
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Note: Module was incomplete. 2 missing questions and others incomplete at the start of phase II.
Q17.4.2
For the ΔG° values given here, determine the standard cell potential for the cell.
- 12 kJ/mol, n = 3
- −45 kJ/mol, n = 1
Q17.4.2
1. Taking a look at the information given, we have ΔG°, and n, which is the number of electrons transferred during the redox reaction. If n was not given directly, we could balance the separate half reactions to find the number of electrons transferred. We know we want to find E°cell, and to relate all of this information we use the equation ΔG°=-nFE°cell, with F as Faraday's constant: 96486 J.
Plugging in our information into the equation we get:
(12kj/mol)=-(3)(96.486kj)(E°cell)
E°cell=-.0414 V
2. Taking a look at the information given, we have ΔG°, and n, which is the number of electrons transferred. We know we want to find E°cell, and to relate all of this information we use the equation ΔG°=-nFE°cell, with F as a constant, 96486 J. Use the same method as above, isolating E°cell.
Plugging in our information into the equation we get:
(-45kj/mol)=-(1)(96.486kj)(E°cell)
E°cell=.466 V
Q12.1.2
Ozone decomposes to oxygen according to the equation 2O3(g)⟶3O2(g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen.
Q12.1.2
We know the rate law equation for the reaction A + B --> C + D is:
\(rate = -\frac{\Delta [Reactant]}{\Delta t} = \frac{\Delta [Product]}{\Delta t}\)
The rate expression relates the decrease in concentration of reactants over time to the increase in concentration of products over time. Also keep in mind that since the reactant is disappearing, there is a negative sign in front of the rate expression. Also, remember to divide each expression by its corresponding stoichiometric coefficient.
To find what the equation that will relate the disappearance of Ozone to the formation of Oxygen, we look at the reaction given and plug in the corresponding coefficients.
\(rate = -\frac{\Delta [O_{3}]}{2 \Delta t} = \frac{\Delta [O_{2}]}{3 \Delta t}\)
Q12.4.20
For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene (CH2=CH–CH=CH2)(CH2=CH–CH=CH2) has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
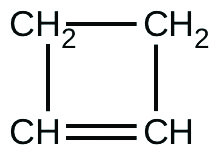
The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0 × 10−4 s−1 at 150 °C in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 °C with an initial pressure of 55 torr.
Q12.4.20
Since this is a first order reaction, the integrated rate law is: \([A_{t}]=[A_{0}]e^{-kt}\)
Partial Pressure: Use the integrated rate law to find the partial pressure at 30 minutes:
Use \(A_0\) = 55 torr, t = 30 min, and k = \(2.0 * 10^{-4}s^{-1}\) to solve the integrated rate law equation:
\([A_{30}]=(55 torr)*e^{-(2.0x10^{-4}\frac{1}{sec})(30min\cdot\frac{60sec}{1 min})}\)
Solve this equation to get:
\([A_{30}]=(55 torr)*e^{-0.36}\)
\(A_{30}]\) = 38.37 torr.
Initial Concentration: Find the initial concentration using the ideal gas law.
The ideal gas law is given by \(PV = nRT → n = \frac{PV}{RT}\). Use this form of the gas law to solve for the initial concentration n.
Use V = 0.53L, R = 0.08206 \(\frac{L*atm}{mol*L}\), T = 423.15 K, and P = \(\frac{1 atm}{760}\) = 0.07237 atm .
Solve the ideal gas equation using these values:
\(n=\frac{(55torr)(0.53L)}{(0.08206\frac{L*atm}{mol*K})(423.15K)} = 0.00110\) moles cyclobutene.
Now find the initial concentration of cyclobutene \(A_0\) using the equation \([A_0] = \frac{n}{V}\):
\(A_0 = \frac{n}{V} = \frac{0.00110 moles}{0.53 L} = 0.00208 M\)
Concentration at 30 minutes: Find the concentration of cyclobutene at 30 minutes by using the integrated rate law given above, using time t = 30 minutes, or 1800 seconds.
\([A_{30}]=(0.00208M)e^{-0.36}= 0.00145M\)
So at 30 minutes, the cyclobutene concentration is 0.00145 M, and the partial pressure is 38.37 torr.
Q21.3.4
Complete each of the following equations:
a. \(^{7}_{3}Li+?\rightarrow2^{4}_{2}He\)
b. \(^{14}_{6}C\rightarrow^{14}_{7}N+?\)
c. \(^{27}_{13}Al+^{4}_{2}He\rightarrow?+^{1}_{0}n\)
d. \(^{250}_{96}Cm\rightarrow?+^{98}_{38}Sr+4^{1}_{0}n\)
Q21.3.4
An alpha particle, α, a positively charged particle consisting of two protons and two neutrons, written as \(_{2}^{4}He\).
Emission by Alpha particles :
- Emitting a alpha particle decreases neutron number by two
- Emitting a alpha particle decreases proton number by two
- Emitting a alpha particle decreases mass number by four
A beta particle β, can either be a positron or an electron. Since the question does not specify, we assume that it is an electron. A beta particle's mass is negligible compared to the mass of a proton or neutron and has a charge of -1. It can be written as \( _{-1}^{0}β\).
Emission by beta particles (negative time = electron) :
- Emitting a negative beta particle decreases neutron number by one
- Emitting a negative beta particle increases proton number by one
- Emitting a negative beta particle does not change mass number
Remember that "nuclear nomenclature" can be written as: \(_{Z}^{A}X\) where
A: Number of nucleons (mass number) Z: The number of protons (atomic number) X: The particle or Element
1. We want to have balanced mass and proton number on the reactants and products side. On the reactants, A = 7 and on the products A = (2x4) = 8. We want the reactants to also equal 8, so A = 1. For the reactants, Z= 3 and for the products Z= (2x2) = 4. This means Z is 1 for the reactants side. This gives us H, since it has mass 1 and atomic number 1.
\(^{7}_{3}Li+^{1}_{1}H\rightarrow2^{4}_{2}He\)
2. With the same method we will balance both the charge and the mass of the reactants and products. On the reactant side we have 14 amu, and on the reactants side we also have 14 amu, so we know that the mass we will add is 0. We have the choices of a positron emission \binom{0}{1}p or gamma ray. Taking a look at the charges, we have 6 on the left and 7 on the right, meaning we need to add a charge to the right. We can then conclude that we will add a positron to the right side to balance the charge
\(^{14}_{6}C\rightarrow^{14}_{7}N+^{0}_{-1}e^{-}\)
3. Once again we will balance the charges and mass. The left side has 31 amu (mass of C + mass of He) and a charge of 15 (charge of C + charge of He) and on the right side we already know we are adding a neutron, which means we will only affecting the mass and not the charge. A neutron has a mass of one, which means the element in question is going to have a mass of one less than the total mass on the left side. The left side has a total mass of 31 amu, so our unknown element will have a mass of 30 amu. We are not changing the charge, so the right side will equal the total charge of the left side, resulting in a charge of 15. Taking a look at the periodic table, with an atomic number of 15 we know the unknown element is Phosphorus.
\(^{27}_{13}Al+^{4}_{2}He\rightarrow^{30}_{15}P+^{1}_{0}n\)
4. We will be adding an element to this equation to balance out the charge and and mass. On the left side we have a total mass of 250, and a toatl charge of 96. On the left side we have the element Strontium , and 4 neutrons, which give a total mass of 102, and a total charge of 38. We know the left side has to add up to 250, and with quick math we know that the mass we will add is 148 and the charge will be 58. Looking at the periodic table and the atomic number 38 we know that we will add Cerium.
\(^{250}_{96}Cm\rightarrow^{148}_{58}Ce+^{98}_{38}Sr+4^{1}_{0}n\)
Q20.1.1
Identify the oxidation state of the atoms in the following compounds:
- PCl3
- CO2-3
- H2S
- S8
- SCl2
- Na2SO3
- SO4-2
Q20.1.1
Here are the oxidation state rules:
-
Rule 1: The oxidation number of an element in its free (uncombined) state is zero — for example, Al(s) or Zn(s). This is also true for elements found in nature as diatomic (two-atom) elements

-
Rule 2: The oxidation number of a monatomic (one-atom) ion is the same as the charge on the ion, for example:


-
Rule 3: The sum of all oxidation numbers in a neutral compound is zero. The sum of all oxidation numbers in a polyatomic (many-atom) ion is equal to the charge on the ion. This rule often allows chemists to calculate the oxidation number of an atom that may have multiple oxidation states, if the other atoms in the ion have known oxidation numbers.
-
Rule 4: The oxidation number of an alkali metal (IA family) in a compound is +1; the oxidation number of an alkaline earth metal (IIA family) in a compound is +2.
-
Rule 5: The oxidation number of oxygen in a compound is usually –2. If, however, the oxygen is in a class of compounds called peroxides (for example, hydrogen peroxide), then the oxygen has an oxidation number of –1. If the oxygen is bonded to fluorine, the number is +1.
-
Rule 6: The oxidation state of hydrogen in a compound is usually +1. If the hydrogen is part of a binary metal hydride (compound of hydrogen and some metal), then the oxidation state of hydrogen is –1.
-
Rule 7: The oxidation number of fluorine is always –1. Chlorine, bromine, and iodine usually have an oxidation number of –1, unless they’re in combination with an oxygen or fluorine.
- Chlorine always has an oxidation of -1, and in this case it is the more electronegative element. Since there is no charge on the overall compound, Phosphorus will have a +3 charge to balance out the 3 chlorines of -1 charge.
- Oxygen (almost) always has a -2 charge and in this case it is the more electronegative element. There are two oxygens giving it a charge of -4, and since the overall compound has a charge of -3, the Carbon will have a charge of +1.
- Hydrogen always has a charge of +1, and since there are two the compound then has a charge of +2. To make the compound neutral, Sulfur will then have a -2 charge.
- S8 is a neutral atom and is an elemental form of sulfur, so it will have a charge of 0.
- Cl2 has a -1 charge multiplied by 2, so Sulfur will have a +2 charge.
- Oxygen has a -2 charge, multiplied by 3 which makes it a -6 charge. Sodium has a +1 charge multiplied by two so it has a +2 charge. This compound is netural, so Sulfur will have to have a +4 charge to balance out the charges.
- Oxygen has a -2 charge multiplied by 4 which is -8, and the overall charge of the compound is -2 so sulfur will have an oxidation state of +6.
Q20.4.19
Carbon is used to reduce iron ore to metallic iron. The overall reaction is as follows:
2Fe2O3⋅xH2O(s)+3C(s)→4Fe(l)+3CO2(g)+2xH2O(g)
Write the two half-reactions for this overall reaction.
Q20.4.19
Half reactions consist of an oxidation reaction and a reduction reaction. These 2 reactions added together yields the redox reaction, where electrons are transferred. To find out which elements go with which reaction, we will take a look at the oxidation numbers. Using the oxidation rules, we will assign oxidation numbers to each element to figure out which one is reduced and which one is oxidized.
- The Fe in Fe2O3 has an oxidation number of +3
- The O in Fe2O3 has an oxidation number of −2
- The H in H2O has an oxidation number of +1
- The O in H2O has an oxidation number of −2
- The C has an oxidation number of 0
- The Fe has an oxidation number of 0
- The C in CO2 has an oxidation number of +4
- The O in CO2 has an oxidation number of −2
- The H in H2O has an oxidation number of +1
- The O in H2O has an oxidation number of −2
We see that Fe is reduced since it goes from +3 to 0, losing charge and gaining electrons. Therefore the reduction half reaction is :
\(Fe_{2}O_{3} \cdot H_{2}O\ +\ 12H^{+}\ +\ 12e^{-}\ \rightarrow\ 4Fe\ +\ 7H_{2}O\)
We see that C is oxidized since it goes from 0 to +4, gaining charge and losing electrons. Therefore the oxidation half reaction is:
\(3C\ \rightarrow\ 3CO_{2}\)
20.9.4
Two solutions, one containing Fe(NO3)2·6H2O and the other containing the same molar concentration of Fe(NO3)3·6H2O, were electrolyzed under identical conditions. Which solution produced the most metal? Justify your answer.
20.9.4
First, observe the these two complexes were electrolyzed under identical conditions. We see that the only difference is that the first complex has 2 nitrate ions with the iron, and the second has 3. Since the complex remains neutral with both of the solutions, this means that the oxidation number of iron is +2 for the first one and +3 for the second one. Since both molecules undergo identical electrolysis, the molecule whose bonds are easier to break will produce the most product. Therefore, since Fe(NO3)2⋅6H2OFe(NO3)2⋅6H2O is more likely to break apart than Fe(NO3)3⋅6H2OFe(NO3)3⋅6H2O, meaning that the Fe(NO3)2⋅6H2O would produce the most metal.
20.8.3
Why is it important for automobile manufacturers to apply paint to the metal surface of a car? Why is this process particularly important for vehicles in northern climates, where salt is used on icy roads?
20.8.3
Paint keeps oxygen and water from coming into direct contact with the metal, which prevents corrosion. Corrosion is a naturally occurring process, and paint creates a boundary layer for the metal, in order to keep oxygen from corroding the metal. Water freezes at 0o, which creates ice. In northern climates that will make the roads very icy if temperatures drop below 0o, but adding salt lowers the freezing point of the water, so the water will need a lower temperature in order to turn to ice. Corrosion is a form of oxidation, and there are many methods to prevent this. Automobile manufacturers spend a great deal of time and money developing paints that adhere tightly to the car’s metal surface to prevent oxygenated water, acid, and salt from coming into contact with the underlying metal.

