Extra Credit 20
- Page ID
- 82989
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.9
- An active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
- An active (metal) electrode was found to lose mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
S17.2.9
Active electrodes participate in the oxidation-reduction reaction. Since metals form cations, the electrode would lose mass if metal atoms in the electrode were to oxidize and go into solution. Oxidation occurs at the anode.
With this in mind, the active metal electrode that gained mass must have been part of the cathode. This is because the metal atoms in the electrode were reduced. Reduction occurs at the cathode. Likewise, the active metal electrode that was found to lose mass must have been part of the anode since this is where oxidation occurs.
An example of this phenomenon is shown in Figure 17.2.9 which shows the mass of the Zinc electrode decreasing (Zn oxidizes into soluble \(Zn^{2+}\)) and the mass of Copper electrode increasing ( \(Cu^{2+}\) reduces into metallic copper which deposits on the copper anode).

Figure 17.2.9
Edit: Yes, I agree with the explanation and the example.
Q19.1.18
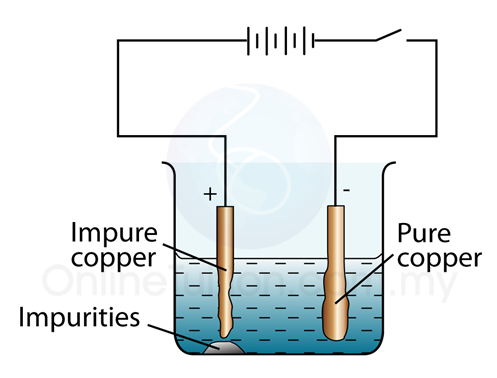
Describe the electrolytic process for refining copper.
S19.1.18
Figure 19.1.18 In an electrolytic cell, oxidation will always occur at the anode and reduction will always occur at the cathode. However, it differs from a galvanic cell because the anode is now a positive charge and cathode is a negative charge.
The electrolytic process for refining copper is to extract the copper through the process of electrolysis. Look at Figure 19.1.18 for a better visual.
The purpose of the electrolytic process to refine copper is to make pure copper. In order to do this, impure copper is made from the anode in an electrolyte bath. When a current is passed through a solution, the copper ions \(Cu^{2+}\) are attracted to the negative cathode, where they gain e- and deposit themselves as neutral copper atoms. Pure copper gets build up at the cathode. At the positive anode, copper atoms give up e- and get dissolved in the solution to form copper ions.
Edit: Yes I agree. An acidic solution of copper sulphate, \(CuSO_4\), can be used as the electrolyte. At anode \(Cu\rightarrow\Cu^{2+}+2e^{-}\) and at Cathode \(Cu^{2+}+2e^{-}\rightarrow\Cu(s)\)
Q19.3.10
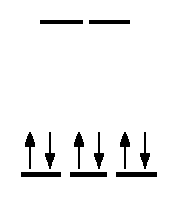
Would you expect the complex \([Co(en)_3]Cl_3\) to have any unpaired electrons? Any isomers?
S19.3.10
The complex does not have any unpaired electrons. The complex does not have any geometric isomers, but the mirror image is nonsuperimposable, so it has an optical isomer.
STEP 1:
Assign oxidation states to each element. Cl- has a -1 oxidation state. En is neutral, so 0. The entire complex is also neutral, so in order to balance the charges out, Co must be +3 because there are 3 chlorides, which gives a -3 charge.
STEP 2:
Write the electron configuration for \(Co^{3+}\). \([Ar]3d^6\). There are 6 electrons.
STEP 3:
Check where en lies on the spectrochemical series. Does it have a strong field strength? It does, so these electrons will exist at the d-level with high splitting energy because the magnitude of the pairing energy is less than the crystal field splitting energy in the octahedral field.
You will the notice that there aren't any unpaired electrons when you draw the Crystal Field Theory (CFT) diagram.
This complex does not have any geometric isomers because cis-trans structures cannot be formed. The mirror image is nonsuperimpoasable, which means the enantiomers are chiral molecules; if the mirror image is placed on top on the original molecule, then they will never be perfectly aligned to give the same molecule.
Edit: Yes, I agree with the well written step by step explanation.
Q12.4.10
The reaction of compound A to give compounds C and D was found to be second-order in A. The rate constant for the reaction was determined to be 2.42 L/mol/s. If the initial concentration is 0.500 mol/L, what is the value of \(t_{1/2}\)?
S12.4.10
Second order half life equation:
$$t_{1/2} = \frac{1}{k[A]_0}$$ ,
where \(t_{1/2}\) is the half-life , k is rate constant, and \([A]_0\).
What are you trying to find?
You are trying to find \(t_{1/2}\).
What are you given?
You are given k=2.42 L/mol/s and \([A]_0\) = 0.500 mol/L.
Now, plug everything in.
Be careful of units. *units cancel out to yield s.
The answer should be 0.826 seconds.
Edit: The order of the reaction is correct as well as the answer for the problem.
Q21.2.5
Write the nuclide notation, including charge if applicable, for atoms with the following characteristics:
- 25 protons, 20 neutrons, 24 electrons
- 45 protons, 24 neutrons, 43 electrons
- 53 protons, 89 neutrons, 54 electrons
- 97 protons, 146 neutrons, 97 electrons
S21.2.5
- \(\ce{^{45}_{25}Mn^{+1}}\);
- \(\ce{^{69}_{45}Rh^{+2}}\);
- \(\ce{^{142}_{53}I^{-1}}\);
- \(\ce{^{247}_{97}Bk}\)
To get atomic mass, add protons and neutrons together. Atomic mass will be located on the upper left-hand side of the element. Atomic number will be located on the lower left-hand side of the element. The atomic number tells you how many protons there are in the element. If the number of protons is equal to the number of electrons, then the element has an overall charge of 0, or is neutral. Figure 21.2.5 shows how to properly format the atomic structure.
Figure 21.2.5
Formula for charge of an element:
$$ Charge = (# of Protons) - (# of Electrons) $$
1. Atomic mass = 25 protons + 20 neutrons = 24
Charge = 25 protons - 24 electrons = +1
2. Atomic mass = 45 protons + 24 neutrons = 69
Charge = 45 protons - 43 electrons = +2
3. Atomic mass = 53 protons + 89 neutrons = 142
Charge = 53 protons - 54 electrons = -1
4. Atomic mass = 97 protons + 146 neutrons = 247
Charge = 97 protons - 97 electrons = 0
Edit: The answers are correct and the step by step instructions are correct.
Q21.5.8
The mass of a hydrogen atom \(\ce{(^1_1H)}\) is 1.007825 amu; that of a tritium atom \(\ce{(^3_1H)}\) is 3.01605 amu; and that of an α particle is 4.00150 amu. How much energy in kilojoules per mole of \(\ce{^4_2He}\) produced is released by the following fusion reaction: \(\ce{^1_1H +3_1H→^4_2He}\).
Q21.5.8
Keep in mind Einstein's famous formula: $$E = mc^2 $$
where E in energy, m is mass, and c is the speed of light \(2.998 \times 10^{8} m/s\). Utilize this formula, though there are slight variations.
$$deltam = (total mass of neutrons and protons) - (mass of the nucleus)$$
Plug in what is given: mass of the nucleus = 4.00150 amu , total mass of neutrons and protons = 1.007825 amu + 3.01605 amu
deltam= (1.007825+ 3.01605) - (4.00150) amu = 0.022375 amu
Now that you have m and c, and you want to find E, plus everything in. Be sure to convert units!
$$E= (.022375 amu) \left( \frac{1g}{6.022 \times 10^{23}}\right) \left(\frac{1 kJ}{1000 J}\right) (2.998 \times 10^{8})^2 $$
$$E =3.34 \times 10^{-12} kJ/mol$$
Edit: This answer is correct.
Q20.4.6
Explain why E° values are independent of the stoichiometric coefficients in the corresponding half-reaction.
Q20.4.6
E standard values do not depend on the stoichiometric coefficients for a half reaction because it is an intensive property, meaning E standard values don't depend on the amount of substance involved. The coefficients in the balanced equation have no effect on the value of the cell potential.
Edit: I agree with this answer. It was said multiple times in class that the balanced equation doesn't always tell you something.
Q20.7.4
Why are galvanic cells used as batteries and fuel cells? What is the difference between a battery and a fuel cell? What is the advantage to using highly concentrated or solid reactants in a battery?
Q20.7.4
Galvanic cells are used to harness the energy (to create a flow of electrons) of spontaneous redox reactions. In order to do so, we have to separate the chemicals of the half-reactions, the anode (where oxidation happens) and the cathode (where reduction happens) which is joined by an apparatus, salt bridge, that allows the electrons to flow from the anode to the cathode (Figure 20.7.4).
This is why galvanic cells are used as batteries and fuel cells: the spontaneous redox reaction supply energy which is used to perform work.
A battery cell and a fuel cell are both galvanic cells, however, a battery cell has all the reactants necessary to produce electricity. On the other hand, a fuel cell requires constant external supply of one or more reactants to generate electricity. It doesn't store chemical or electrical energy.
The advantage to using highly concentrated or solid reactants in a battery is that the concentration of the reactants and products don't change greatly as the battery is discharged. During the discharge process, output voltage pretty much stays constant.

Figure 20.7.4 This is the basic structure in batteries and fuel cells.
Edit: Correct and well thought out explanation.