Extra Credit 15
- Page ID
- 82983
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.4 Balance the following reactions and write the reaction using cell notation. Ignore any inert electrodes, as they are never part of half reactions.
a) Al(s) + Zr4+(aq)→ Al3+(aq)+Zr(s)
First, write the the oxidation and reduction half reaction equations
Oxidation: Al(s) → Al3+(aq)
Reduction: Zr4+(aq) → Zr(s)
Then, balance each reaction using electrons to obtain an equal charge on both sides of the equation
Al(s) → Al3+(aq) + 3e-
Zr4+(aq) + 4e- → Zr(s)
Multiply each equation by a number to obtain a common factor between the number of electrons. In this case, one would multiply the oxidation equation by 4 and the reduction equation by 3 to get a common factor of 12 electrons.
4Al(s) → 4Al3+(aq) + 12e-
3Zr4+(aq) + 12e- → 3Zr(s)
Combine the two half-equations and cross-cancel the items that are repeated on each side of the equation.
4Al(s) → 4Al3+(aq) + 12e-
3Zr4+(aq) + 12e- → 3Zr(s)
final equation: 4Al(s) + 3Zr4+(aq) → 4Al3+(aq) + 3Zr(s)
The above equation is the final, balanced equation. When checking your work, notice that both sides of the equation must be equal in charge.
The cell notation is written as follows:
Al(s) | Al3+ (aq) || Zr4+ (aq) | Zr(s)
A single bar indicates a phase change and a double bar indicates a salt bridge. The anode (where oxidation occurs) is the left most part of the diagram, while the cathode (where reduction occurs) is the right most part of the diagram. Keep ions next to their solid counterparts, as the single bar indicates a change in phase from solid to aqueous solution. One can add an inert electrode if needed, which is an electrode low in reactivity, and is used if the anode/cathode is not a solid.
b) Ag+(aq) + NO(g) → Ag(s) + NO3-(aq)
Again, write each half reaction equation
Ag+(aq) → Ag(s)
NO(g) → NO3-(aq)
Balance each half reaction. This time, instead of just electrons, one must also balance the elements. Start with oxygen. Add water molecules to balance the number of oxygens on each side of the equation. Then, to balance out the hydrogens, add H+ ions to each side of the equation.
Ag+(aq) + e- → Ag(s)
NO(g) + 2H2O(l) → NO3-(aq) + 4H+(aq) + 3e-
Multiply by the lowest common multiple and combine the two equations.
3Ag+(aq) + 3e- → 3Ag(s)
NO(g) + 2H2O(l) → NO3-(aq) + 4H+(aq) + 3e-
final equation: 3Ag+(aq) + NO(g) + 2H2O(l) → 3Ag(s) + NO3-(aq) + 4H+(aq)
Notice: the charges are equal on both sides of the equation
Using the same rules from part a, the cell notation is as follows:
Pt(s) l NO(g) l NO3-(aq) ll Ag+(aq) l Ag(s)
We had to use an inert electrode here because NO is a gas.
c) SiO32-(aq) + Mg(s) → Si(s) + Mg(OH)2(s) {in basic solution}
This equation starts off the same as part b, but because it is in basic solution, one must add an OH- to each side of the equation for as many H+ are added.
SiO32-(aq) + 6H+(aq) + 6OH-(aq) +4e- → Si(s) +3H2O(l) + 6OH-(aq)
Mg(s) +2H2O(l) + 2OH-(aq) → Mg(OH)2 + 2H+(aq) + 2OH-(aq)+ 2e-
Multiply by the lowest common multiple and combine the two equations, then cross cancel any items that appear on both sides. Notice how the H+ and OH- on the same sides of the equations turn into water when combined.
SiO32-(aq) + 6H+(aq) + 6OH-(aq) +4e- → Si(s) +3H2O(l) + 62OH-(aq)
2Mg(s) +4H2O(l) + 4OH-(aq) → 2Mg(OH)2 + 4H+(aq) + 4OH-(aq)+ 4e-
final equation: SiO32-(aq) + 2Mg(s) + 3H2O(l) → Si(s) + 2Mg(OH)2 + 2OH-
The cell notation, continuing to follow the same rules from part a, is as follows:
Mg(s) l Mg2+(aq) ll SiO32-(aq) l Si(s)
d) ClO3-(aq) + MnO2(s) → Cl-(aq) + MnO4-(aq) {basic}
Treat this problem the exact same way as part c. Start by splitting the equation into its two half reactions, then balance each side by elements first, then charge.
ClO3-(aq) +6H+(aq) + 6OH-(aq) + 6e- → Cl-(aq) + 3H2O(l) + 6OH-(aq)
MnO2(s) + 2H2O(l) + 4OH-(aq) → MnO4-(aq) + 4H+(aq)+ 4OH-(aq)+ 4e-
Next, combine the two equations (multiply the first by 2 and the second by 3) to make one final, balanced equation.
2ClO3-(aq) + 12H+(aq) + 12OH-(aq) + 12e- → 2Cl-(aq) + 6H2O(l) + 12OH-(aq)
3MnO2(s) + 6H2O(l) + 12OH-(aq) → 3MnO4-(aq) + 12H+(aq)+ 12OH-(aq)+ 12e-
final equation: 2ClO3-(aq) + 3MnO2(s) → 3MnO4-(aq) + 2Cl-(aq)
The cell notation of this reaction is:
MnO2(s) l MnO4-(aq) ll ClO3-(aq) l Cl-(aq) l Pt(s)
Notice the use of the inert electrode at the cathode. This is because Cl- is aqueous.
Q19.1.13 Find the potentials of the Electrochemical Cell below.
Cd l Cd2+(0.1 M) ll Ni2+(0.5M) l Ni
For this problem, we must find Ecathode and Eanode in order to find Ecell.
The Cathode is where reduction occurs. The cathode half reaction is:
Ni2+(aq) + 2e- → Ni(s)
By looking at a chart of standard reduction potentials, we find that the standard reduction potential of the reduction half reaction, Ecathode, to be -0.26V
The Anode is where oxidation occurs. The anode half reaction is:
Cd2+(aq) + 2e- → Cd(s)
You might be thinking: why is it written as a reduction equation, even though this is the oxidation half reaction? For exercises such as these, we are looking for the standard reduction potential. Thus, all the equations are written in the reduced form. By looking at the chart of standard reduction potentials, we find that Eanode is -0.4V
The equation to combine these two values is:
Ecell = Ecathode - Eanode
Ecell = (-0.26V) - (-0.4V) = 0.14V
Q19.3.5 Is it possible for a complex of a metal in the transition series to have six unpaired electrons?
It is possible for a complex of a metal in the transition series to have six unpaired electrons. This occurs when a metal with 5 electrons in the d-subshell loses an electron from the s-subshell. For example, Mn+ is one such element. Mn with no charge has five valence electrons. However, by transition metal rules, it loses its first two electrons from the 4s subshell before it loses electrons from the 3d subshell. This makes its electron configuration [Ar]4s13d5. This same concept applies to Tc+ and Re.
Q12.4.5 Use the graphical method to determine the order and rate constant of the equation below.
2X → Y + Z
| Time (s) | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 |
|---|---|---|---|---|---|---|---|---|
| [X] (M) | 0.0990 | 0.0497 | 0.0332 | 0.0249 | 0.0200 | 0.0166 | 0.0143 | 0.0125 |
If you put these numbers in an excel program or a graphing calculator, you come out with a negative exponential shaped graph. This means the reaction is second order, because second order reactions have an exponential shape in time v. concentration graphs.
The rate is determined by the equation:
d[X]/dt
This means the change in the concentration of X divided by the change in time. By plugging in numbers from the chart, we get the following result:
(0.0125 - 0.0990)/(40-5) = -0.00247
Because the rate is positive, the answer to the rate is 0.0027 M/s
Q12.7.8 Based on the Diagrams in 12.7.6, which of the reactions has the fastest rate? The slowest? Based on the diagrams in 12.7.7, which of the reactions has the fastest rate? The slowest?
To solve both of these questions, one would be looking for the reaction with a smaller activation energy. The reaction with a smaller activation energy will have the fastest rate. Activation energy is the energy needed for a reaction to occur. This is determined by subtracting the energy of the reactants from the energy of the peak of the graph.
Graphs from 12.7.6
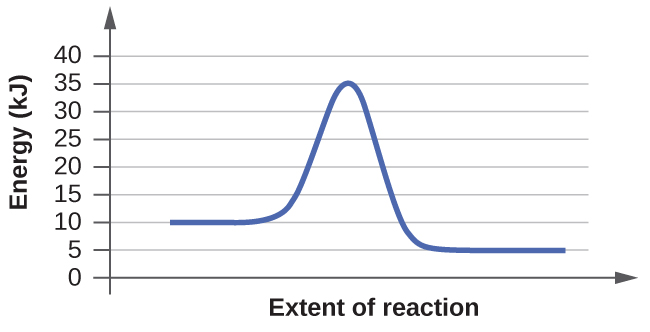
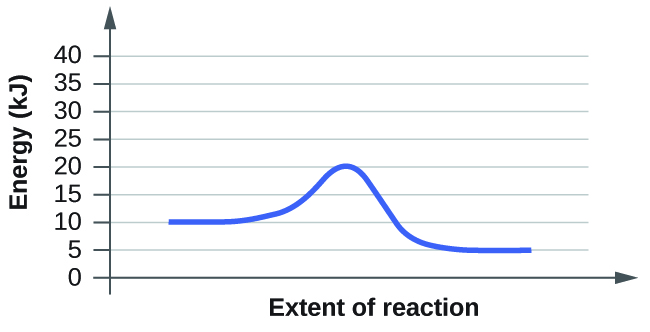
The right diagram has the faster rate. This is because it has a lower activation energy.
The left graph has 10 kJ of energy for the reactants and 35kJ of energy at the peak.
35kJ - 10kJ = 25 kJ = Activation Energy
The right graph has 10 kJ of energy for the reactants and 20kJ of energy at the peak.
20kJ - 10kJ = 10 kJ = Activation Energy
Because 10kJ is less than 25kJ, the right graph has a lower activation energy and thus a faster rate.
Graphs from 12.7.7
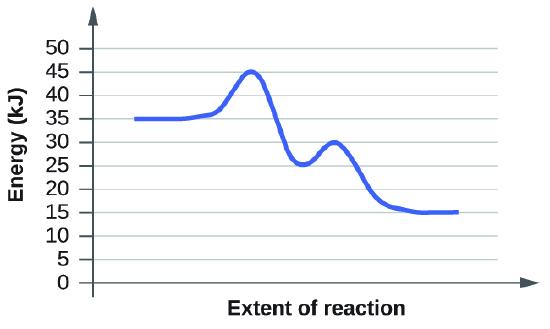
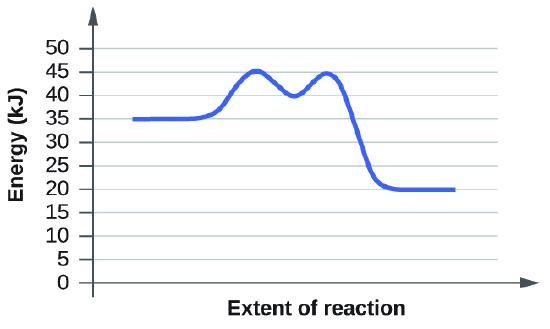
Both graphs have equal activation energy, and thus have an equal rate. The peaks of each graph is 45kJ, and the reactants' energies both begin at 35 kJ. The difference in both cases is 10kJ, giving them equal rates of reactions.
Q21.5.3 Both fusion and fission are nuclear reactions. Why is a very high temperature required for fusion, but not fission?
Although fusion and fission are both high energy nuclear reactions, they differ in one key principle.
Fission is the splitting of a heavy, unstable nucleus into two lighter nuclei.
Fusion is the combination of two lighter nuclei, which releases a large quantity of energy.
This difference is crucial in the comprehension of this question. The high temperature is required for fusion, but not fission, because the two lighter particles must have enough energy to combine in the process of fusion. In order for the two molecules to collide, they must have a high enough kinetic energy to overcome the positive force that naturally repels them. This increase in kinetic energy can be obtained with high temperatures. Because fission does not involve two molecules colliding, it does not need to happen at very high temperatures.
Q20.4.1 Is a hydrogen electrode chemically inert? What is a major disadvantage to using a hydrogen electrode?
A hydrogen electrode is not chemically inert. Hydrogen is used as the standard electrode (the standard hydrogen electrode, SHE). Scientists use it as a reference for all half-reaction cell potentials. It's value is zero on the list of standard reduction potentials, which is not that low on the list of standard reduction potentials. Because it is used as a comparison and its value is zero, it is not inert. Hydrogen is still reactive in redox reactions and can play a chemical role. Some examples of chemically inert electrodes are Pt and C(graphite). This is because they are so low on the list of standard reduction potentials that they react with very few elements on the list.
Q20.5.30 Hydrogen gas reduces Ni2+ according to the reaction below:
Ni2+(aq) + H2(g) → Ni(s) + 2H+ {E°cell = -0.25V (delta)H = 54kJ/mol}
a) What is k
To find k, we need to break out the Big Triangle
(delta)G° = -RTlnK = -nFE°cell
We also need to know how many moles of electrons are transferred in this redox reaction. Do this by writing the half reactions.
Ni2+(aq) + 2e- → Ni(s)
H2(g) → 2H+(aq) + 2e-
Given this, we can conclude that n=2 moles of electrons transferred. Plug in the numbers given above into The Big Triangle equation. Assume 298 K and Farraday's constant is 96485J/Vmol.
-(8.314J/Kmol)(298K)(lnk) = -(2mol)(96485J/Vmol)(-0.25V)
k=3.496x10-9
b) Is this reaction likely to occur?
This reaction is unlikely to occur. We can conclude this by looking at the k value we found in part a and the (delta)H value given to us in the problem. Reactions likely to occur have large k values and negative (delta)H values. Because we have neither of these scenarios, we can conclude that this reaction is unlikely to occur.
c) What conditions could be changed to increase the likelihood of this reaction happening as written?
Because the problem says "as written," we cannot simply say the reverse reaction would increase the likelihood of it occurring. Although this is true, that changes the writing of the equation. To increase the likelihood of this happening as written, we can increase the temperature. Increasing the temperature increases the k value, resulting in a greater likelihood of this reaction naturally occurring.
d) Is this reaction more likely to occur in a high or low pH?
Because H+ is a product of this reaction, decreasing the pH would increase the amount of H+ ions in the products. By le Chatelier's principle, this shifts the reaction left, resulting in an increase of the products. For this reason, this reaction is more likely to occur in a high pH. We want an increase in products, not reactants. In order for that to happen, it would have to be in a high pH.

