Extra Credit 14
- Page ID
- 82982
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
17.2.3 Solutions
Question: For the cell notations in the previous problem, write the corresponding balanced reactions.
a) \(Mg(s)|Mg^{2+}(aq)||Cu^{2+}(aq)|Cu(s)\)
If we look at the cell notation, we can break this down into two half reactions: an oxidation reaction and a reduction reaction. Now we know the anode is always written on the left side of the cell notation. The anode is also always the oxidized substance. Therefore we know the Magnesium metal is being oxidized and the Copper (II) ion is being reduced. Let's break this into the two half reactions and be sure to balance each with the appropriate amount of electrons:
Oxidation Half-Reaction:
\[Mg(s){\rightarrow} Mg^{2+}(aq)+2e^- \]
Reduction Half-Reaction:
\[2e^-+Cu^{2+}(aq)\rightarrow Cu(s)+2e^-\]
Notice that the stoichiometry of the electrons will allow them to cancel out. If the stoichiometric coefficient of the electrons were not the same in the two half-reactions, we would have to multiply one or both of the half reactions by a factor until we had a common electron coefficient.
Now, we can add both half reactions to come to our solution.
\[=Cu^{2+}(aq)+Mg(s)\rightarrow Cu(s)+Mg^{2+}(aq)\]
b) \(Ni(s)|Ni^{2+}(aq)||Ag^+(aq)|Ag(s)\)
We can go through this cell notation with a very similar approach as problem "a". We know the oxidation reaction is going to be Nickel losing electrons because it is on the left side of the cell notation. Thus, the Silver ion is being reduced. Let's write the half reaction for each of the substances.
Oxidation Half-Reaction:
\[Ni(s){\rightarrow}Ni^{2+}(aq)+2e^-\]
Reduction Reaction:
\[e^-+Ag^+(aq)\rightarrow Ag(s)\]
In this problem, we can see the coefficient of the electrons is not equal. Before we add the two reactions together, we need to multiply the entire reduction half-reaction by a factor of 2.
\[2(e^-+Ag^+(aq)\rightarrow Ag(s)\]
Now, we can add the two half-reactions together.
\[Ni(s){\rightarrow}Ni^{2+}(aq)+2e^-\]
\[+\]
\[2e^-+2Ag^+(aq)\rightarrow 2Ag(s)\]
\[=2Ag^+(aq)+Ni(s){\rightarrow}Ni^{2+}(aq)+2Ag(s)\]
19.1.12
Question: How many cubic feet of air at a pressure of 760 torr and 0 °C is required per ton of Fe2O3 to convert that Fe2O3 into iron in a blast furnace? For this exercise, assume air is 19% oxygen by volume.
19.1.12 Solutions:
This question uses a series of unit conversions and the \(PV=nRT\) equation.
The first step is to write out the balanced chemical equation for the conversion of Fe2O3 to pure iron.
\[2\;Fe_2O_3(s)\rightarrow 4\;Fe(s)+3\;O_2(g)\]
Next, we need to analyze the original question to determine the value that we need to solve for. Because the question asks for a value of cubic feet, we know we need to solve for volume. We can manipulate \(PV=nRT\) to solve for volume.
\[V={nRT}/P\]
Now determine the known variables and convert into units that will be easy to deal with.
\[n = 2000\:lbs\; Fe_2O_3\frac{453.592\: grams\: Fe_2O_3}{1\: lb \:Fe_2O_3}\frac{1\: mole \:Fe_2O_3}{159.69\: grams \:Fe_2O_3}\frac{3 \;moles\: O_2}{2\; moles \:Fe_2O_3}\]
\[n=8521\: moles\: of \:O_2\]
Convert to atm for easier calculations
\[R=\frac{.0821\:L\:atm}{mol\:K}\]
\[T=0^{\circ}C=273\:K\]
\[P=760 \:torr= 1 \:atm\]
Now plug the numbers into the manipulated gas law to get to an answer for V.
\[V=190991.8\: liters \:of\: O_2\]
From here we convert liters to cubic feet.
use the conversion
\[1\;L=.0353 ft^3\]
thus we have 6744.811 ft3 of O2
We then refer back to the initial question and remember that this value is only 19% of the volume of the total air. So use a simple equation to determine the total volume of air in cubic feet.
\[6744.811ft^3=.19x\]
x=35499 ft3 of air
19.3.4
Question: The solid anhydrous solid CoCl2 is blue in color. Because it readily absorbs water from the air, it is used as a humidity indicator to monitor if equipment (such as a cell phone) has been exposed to excessive levels of moisture. Predict what product is formed by this reaction, and how many unpaired electrons this complex will have.
19.3.4 Solutions:
From our knowledge of ligands and coordination compounds (or if you need a refresher Coordination Compounds), we can assume the product of CoCl2 in water. H2O is a common weak field ligand that forms six ligand bonds around the central Cobalt atom while the Chloride stays on the outer sphere. We can use this to determine the complex:
\([Co(H_2O)_6]Cl_2\)
From this formation, we can use the Crystal Field Theory (CFT)(Crystal Field Theory) to determine the number of unpaired electrons. This coordination compound has six ligand bonds attached to the central atom which means the CFT model will follow the octahedral splitting. Keep in mind that we know H2O is a weak field ligand and will produce a high spin. High spin is when the electrons pairing energy (P) is greater than the octahedral splitting energy. Thus, the electrons spread out and maximize spin.
In order to fill out our crystal field diagram, we need to determine the charge of cobalt. Because the H2O ligand is neutral, and there are two chlorine ions, we can deduce the charge of cobalt is plus two in order to make the coordination complex neutral. From here, we can use the electron configuration of Co2+ is [Ar]4s23d7. The electrons that are taken away from the cobalt atom in order to form the plus two charge will from the 4s orbital and leave the 3d orbital untouched. Thus, there will be 7 electrons in the crystal field diagram and appear as:
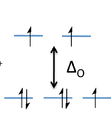
We can see here that there are 3 unpaired electrons.
12.4.4
Pure ozone decomposes slowly to oxygen, \(2O_3(g)\rightarrow3O_2(g)\). Use the data provided in a graphical method and determine the order and rate constant of the reaction.
| Time (h) | 0 | 2.0 × 103 | 7.6 × 103 | 1.23 × 104 | 1.70 × 104 | 1.70 × 104 |
|---|---|---|---|---|---|---|
| [O3] (M) | 1.00 × 10−5 | 4.98 × 10−6 | 2.07 × 10−6 | 1.39 × 10−6 | 1.22 × 10−6 | 1.05 × 10−6 |
12.4.4 Solutions:
We can use the integrated rate law to derive three different graphs to determine order based on the slope of the line. The three graphs that help us determine chemical order are concentration [A] v. time, ln[A] vs. time, and 1/[A] vs. time. If the slope of [A] vs. time is a negative line, we can conclude the reaction is zeroth order. If the graph ln[A] vs. time is a negative line, we can conclude it is a first order reaction. If the graph of 1/[A] vs. time forms a positive sloping line, we can conclude the reaction is second order
![graph 1244. [a] v time.png](https://chem.libretexts.org/@api/deki/files/112024/graph_1244._%255Ba%255D_v_time.png?revision=1&size=bestfit&width=475&height=285)
![screen shot of ln[A] v time.png](https://chem.libretexts.org/@api/deki/files/112026/screen_shot_of_ln%255BA%255D_v_time.png?revision=1&size=bestfit&width=618&height=355)

If you graph all of the previously mentioned graphs, we can pick out this graph (above) because it is the only linear trend, and conclude this reaction is second order.
In order to determine k for the graph, we have to look at the slope of the line.
The k value can be calculated by determining slope. In this case:
\[K=46.37M^{-1}\:hour^{-1}\]
12.7.7
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
12.7.7 Solutions:
(a)
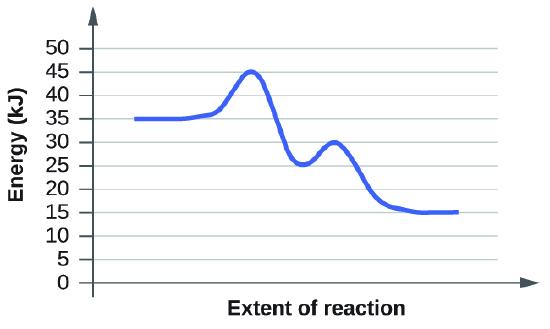
In order to determine activation, we can draw an imaginary line that goes tangent to the reactants (horizontal line at 35 kj) and another tangent line at the "hump" or transition state (horizontal line at 45 kj). We can take the difference between these two tangent lines to determine the activation energy of the first step of the reaction.
Ea of the first step in the reaction: \[45kJ-35kJ=10kJ\]
In order to find the to find the activation energy of the second step in the reaction, we go through a similar process. Take a tangent line at the intermediate or trough in the graph (horizontal line at 25kJ). Add another tangent line at the second transition state (horizontal line at 30kJ). Take the difference of the two tangent lines to determine the second activation energy.
Ea of the second step in the reaction: \[30kJ-25kJ=5kJ\]
In order to determine the overall activation energy, take the difference between the greatest energy tangent line and the initial reactants.
Overall Ea: \[45kJ-35kJ=10kJ\]
(b)
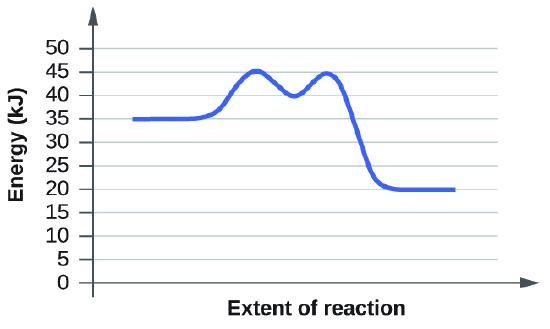
This undergoes a very similar process of taking tangent lines.
Ea of the first step in the reaction: \[45kJ-35kJ=10kJ\]
Ea of the second step in the reaction: \45kJ-40kJ=5kJ]\
Overall Ea : \[45kJ-35kJ=10kJ\]
21.5.2
How does nuclear fission differ from nuclear fusion? Why are both of these processes exothermic?
21.5.2 Solutions:
Nuclear Fusion and Nuclear Fission are easily mistaken for each other. The nuclear processes are very different, however, they both result in a nuclear change and release energy (exothermic). Nuclear Fusion occurs when two nuclei collide to form one heavier atom whereas nuclear fission when one heavier atom splits into two atoms. For nuclear fusion to occur atoms must be at very high temperatures and high pressures. The sun satisfies both these conditions and is a common place for nuclear fusion. Fission's splitting process is a chain reaction which occurs to continue an atomic splitting process. Fission occurs when the nucleus is bombarded with neutrons.

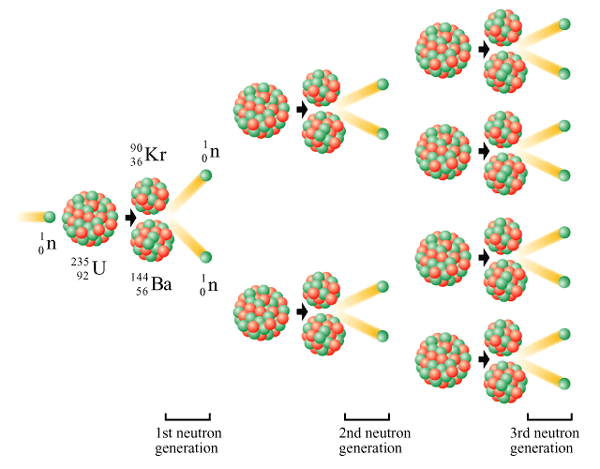
The photo on the left illustrates the difference between nuclear fission and nuclear fusion. The photo on the right illustrates a nuclear fission chain reaction.
20.3.16
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
20.3.16 Solutions:
We can determine if the reaction is spontaneous through this equation:
\[E^{\circ}Cell=E^{\circ}Cell_{Cathode}-E^{\circ}Cell_{Anode}\]
If the EoCell value is positive, the reaction is spontaneous.
1.Co(s) + Fe2+(aq) → Co2+(aq) + Fe(s)
\[Co(s)|Co^{2+}(aq)||Fe^{2+}(aq)|Fe(s)\]
We know the right side of the cell notation represents the cathode and the left is the anode. If we look up the standard reduction potentials for Cobalt (II) and Iron (II), we get:
\[-0.17V=-0.45V-(-0.28V)\]
The EoCell is a negative value. Thus, the reaction is non-spontaneous.
So we must rearrange the cell diagram to compute a positive value and spontaneous reaction. We can switch what is being oxidized and reduced which will change the Eocell value.
\[Fe(s)|Fe^{2+}(aq)||Co^{2+}(aq)|Co(s)\]
Then we get:
\[0.17V=(-0.28V)-(-0.45V)\]
which is a positive value, therefore, this cell diagram is spontaneous.
2. O2(g) + 4H+(aq) + 4Fe2+(aq) → 2H2O(l) + 4Fe3+(aq)
We can go through the same process as above of determining what is being reduced and oxidized. Find the standard reduction potential (SRP) value of Iron and hydrogen/oxygen.
We can see Iron (II) is being oxidized and the oxygen and hydrogen are being reduced.
After we get the SRP values we can use the E0cell equation to determine spontaneity.
The SRP for the hydrogen/oxygen reaction is 1.23 Volts and occurs at the cathode because both are being reduced. The SRP for Iron is .77 and occurs at the anode.
\[0.46V=1.23V-0.77V\]
This value is positive, so we can set up a cell diagram with iron on the left side (anode) and hydrogen/oxygen on the right side (cathode).
However, when we go to set up the cell diagram, we realize there is no solid that would act as an electrode and supply electrons for the iron reaction. So we will need an inert electrode to be the anode. The case is the same at the cathode due to the heterogeneity of the reaction and lack of a solid. Both sides will have inert electrodes. In this case we will use Platinum.
\[Pt(s)|Fe^{2+}(aq),Fe^{3+}(aq)||O_2(g)|H^+(aq)|H_2O(l)|Pt(s)\]
3. 6Hg2+(aq) + 2NO3−(aq) + 8H+ → 3Hg22+(aq) + 2NO(g) + 4H2O(l)
In this reaction, we need to assign every atom with an oxidation number and compare how the specific atom changes to determine what atoms are being oxidized and reduced. In this case, we can track the oxidation number of Mercury. Mercury goes from +2 to +1 thus Mercury gains and electron and is reduced (cathode). The nitrogen atom gets oxidized as it goes from a +5 to a +2. This means the Nitrogen reaction occurs at the anode.
The SRP for the Mercury half reaction is .911 Volts. The SRP for the Nitrate half reaction is .96. We can use the E0Cell equation to determine spontaneity.
\[-0.05V=0.91-0.96V\]
The current reaction is non-spontaneous. in order to make the reaction spontaneous, we need to oxidize the Mercury and reduce the Nitrogen. In this case, the cell diagram will look like this and generate a positive EoCell value and be a spontaneous reaction.
We will need two inert electrodes.
\[Pt(s)|Hg^{2+}(aq),Hg_2^{2+}(aq)||NO_3^-(aq)|NO(g)|H_2O(l)|Pt(s)
From here we get a positive EoCell value.
\[0.05V=0.96V-0.91V\]
4. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
First, we need to track the transfer of electrons and determine what is being oxidized and reduced. If you give every atom an oxidation number and compare its oxidation with the products, we can determine what is being oxidized and reduced. In this case, Carbon goes from a -4 to a +4 and Oxygen goes from 0 to -2.
The SRP for the carbon oxidation is -0.25. The SRP for the Oxygen reduction is .82.
Thus, we can set up an EoCell equation to determine spontaneity.
\[1.07V=0.82V-(-0.25V)\]
This value is positive so we can setup our cell diagram based on the following conclusions. We will need two inert electrodes.
\[Pt(s)|CH_4(g),CO_2(g)||O_2(g),H_2O(g),CO_2(g)|Pt(s)\]
20.5.29
You have constructed a cell with zinc and lead amalgam electrodes described by the cell diagram Zn(Hg)(s)∣Zn(NO3)2(aq)∥Pb(NO3)2(aq)∣Pb(Hg)(s). If you vary the concentration of Zn(NO3)2 and measure the potential at different concentrations, you obtain the following data:
| Zn(NO3)2 (M) | Ecell (V) |
|---|---|
| 0.0005 | 0.7398 |
| 0.002 | 0.7221 |
| 0.01 | 0.7014 |
20.5.29 Solutions:
- Write the half-reactions that occur in this cell.
We can determine the half reactions by following the reaction seeing what is oxidized and reduced. In this case, Zinc is losing electrons (oxidized) and Lead is being reduced (gaining electrons). Write each of the balanced half reactions:
Oxidation half-reaction:
\[Zn(s){\rightarrow}Zn^{2+}(aq)+2e^-\]
We did not include the Mercury and nitrate because they are not being oxidized nor reduced.
Reduction Half Reaction:
\[2e^-+Pb^{2+}(aq){\rightarrow}Pb(s)\]
2. What is the overall redox reaction?
The overall redox reaction can be obtained by adding the two half reactions together. The coefficient of the electrons are the same and are on opposite sides of the reaction so they cancel and are not necessary for the overall redox reaction.
\[Zn(s)+Pb^{2+}(aq){\rightarrow}Zn^{2+}(aq)+Pb(s)\]
3. What is E°cell? What is ΔG° for the overall reaction?
To determine the Eo cell, we will need to look up the standard reduction potentials. The values in the table are not at standard concentration conditions (1M).
Reduction potential for the Zinc reaction is:
Zn EoCell= -0.76V
The Lead Reduction Potential is:
Pb EoCell= -0.13V
Now to determine Eo Cell for the overall reaction:
\[E^{\circ}Cell=E^{\circ}Cell_{Cathode}-E^{\circ}Cell_{Anode}\]
We can look at our redox half-reactions to determine that the Zinc is at the anode because it is being oxidized and Lead is at the cathode because it is being reduced. Thus,
\[E^{\circ}Cell=\:(-0.13V)\:-\:(-0.76V)\]
\[E^{\circ}Cell=0.63V\]
With Eo Cell we can determine the change in standard Gibbs free energy through the equation
\[\Delta{G}=-nFE^{\circ}Cell\]
The n value represents the moles of electrons transferred. We can look at our balanced half reactions above to determine this value. Because both reactions transfer 2 moles of electrons, our n value is 2. If the moles of electrons differed between the two half-reactions, we would have to multiply the reactions be a coefficient until we got the same electron coefficient. F is Faraday's constant.
\[\Delta{G}=(-2\:\frac{mole\:e^-}{mole\:reaction})(\frac{96.485\:kJ}{(Volt)\:\:(mole\:of\:e^-)})(0.63\:Volts)\]
\[\Delta{G}=\:-121.59\:\frac{kJ}{mole\:of\:reaction}\]
4. What is the equilibrium constant for this redox reaction?
To determine the equilibrium constant, we will use:
\[E^{\circ}Cell=\frac{RT}{nF}lnK\]
We know the EoCell value, the temperature (285 K because at standard conditions), Faradays' constant, the gas constant R (in this case we will use 8.314 J K-1mol-1 ) and the number of moles of electrons transferred. We need to manipulate the equation and solve the equilibrium constant (K).
Plug all of these numbers into the equation above and solve.
\[.63=.01284\ln{K}\]
Now, divide each side by .01284.
\[49.065=\ln{K}\]
To solve for K, we will need to make each side of the equation an exponent of e.
\[e^{49.065}=e^{\ln{K}}\]
We can see the \(e^{\ln{K}}\) will just equal K due to basic natural logarithms rules.
Thus,
\[K=2.036 x 10^{21}\]
*In this case, "K" is unitless because it is an equilibrium constant. Be careful when dealing with "k" in equilibrium and "k" in rate laws. For rate laws, "k" has units!!!

