Extra Credit 11
- Page ID
- 82979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.10
Why must the charge balance in oxidation-reduction reactions?
Solution Q17.1.10
The charge must be balanced because the electron lost in oxidation half reaction is gained by reduction half reaction. There is always no lost or creation of electrons, oxidation and reduction must occur simultaneously.
Q19.1.9
Why is the formation of slag useful during the smelting of iron?
Solution Q19.1.9
Slag is useful because it can form a protective coat that prevents iron from oxidizing back to its original reactant, Fe2O3.
Q19.3.1
Determine the number of unpaired electrons expected for [Fe(NO2)6]3−and for [FeF6]3− in terms of crystal field theory.
Solution Q19.3.1
Both NO2 and F have a charge of -1 and each of these complexes has a charge of -3. Fe then must have a charge of +3 so that 3+6(-1)=-3, which is the charge of the entire complex. If we look at the periodic table, Fe has eight electrons when it has zero charge, so Fe3+ has 5 electrons that we need to put on its energy level diagram.
The spectrochemical series tells us whether a ligand will be high spin or low spin: $$\mathrm{\underset{\textrm{strong-field ligands}}{CO\approx CN^->}NO_2^->en>NH_3>\underset{\textrm{intermediate-field ligands}}{SCN^->H_2O>oxalate^{2-}}>OH^->F>acetate^->\underset{\textrm{weak-field ligands}}{Cl^->Br^->I^-}}$$
[Fe(NO2)6]3−: NO2 is a strong ligand field, so it will be low spin, and therefore it has 1 unpaired electron.
[FeF6]3−: F is a weak ligand field, so it will be high spin, and therefore it has 5 unpaired electrons.


Low spin means that it is easier for the electrons to pair up than live in the eg orbitals, giving a electron configuration like the first picture. High spin means that the pairing energy is larger than the crystal field splitting energy, meaning that the electrons will occupy both t2g and eg orbitals before pairing up.
Q12.4.1
Describe how graphical methods can be used to determine the order of a reaction and its rate constant from a series of data that includes the concentration of A at varying times.
Solution Q12.4.1
Graphs can simply show the relationship between [A], ln[A], or 1\[A] and time. When the graph displays a straight line, or is close to a straight line, it will help us determine the order of the reaction.
- If the graph of [A] vs. time is linear, then the reaction is zero order because the integrated rate law of a zero order reaction is [A]=[Ao]-kt.
- If the graph of ln[A] vs. time is linear, then the reaction is first order because the integrated rate law of a first order reaction is ln([A]/[Ao])=-kt.
- If the graph of 1/[A] vs. time is linear, then the reaction is second order because the integrated rate law of a second order reaction is 1/[A]=kt+1/[Ao].
The integrated rate laws work like equations of a line: [A], ln[A], and 1/[A] are all equal to y and t is x if one compares these equations to the line equation y=mx+b. This also means that the rate constant of the reaction, k, is the slope of these graphs, so you can graph experimental data and find the rate constant by finding the slope of the line.
Q12.7.4
For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed:
Solution Q12.7.4
A catalyst increases the rate of a reaction and lowers its activation energy. Graphically, a catalyzed reaction will not be as tall as an uncatalyzed reaction (if the y-axis represents energy) because it takes less energy to start the reaction.
(a)
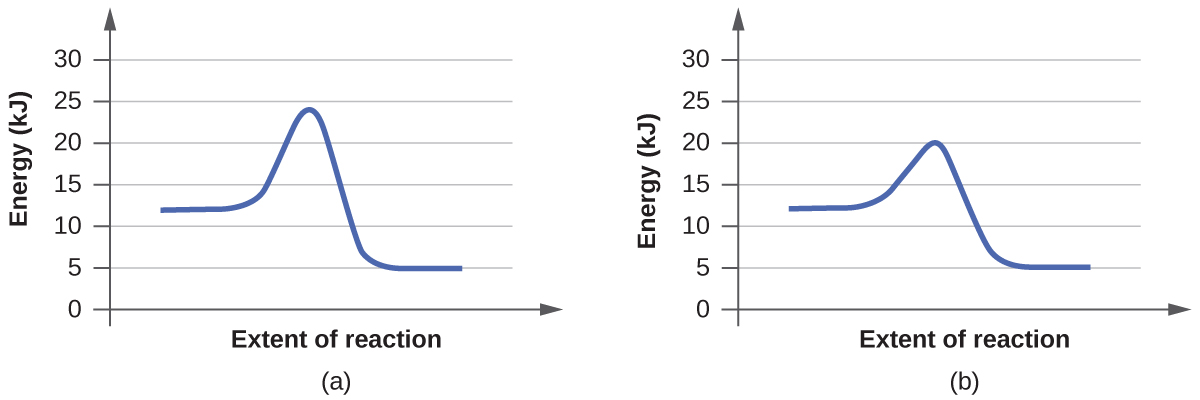
Graph (b) is catalyzed because it passes through an alternative pathway with a lower activation energy barrier.
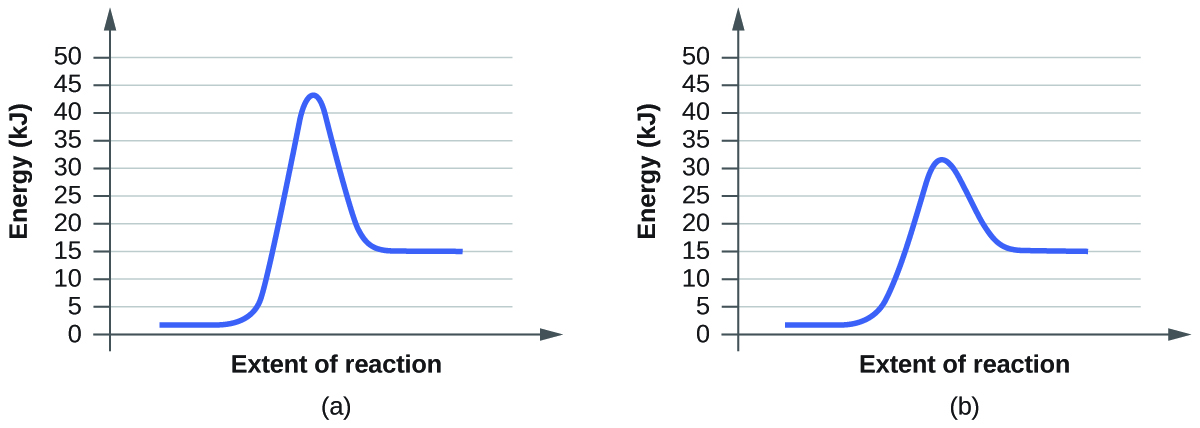
Here, graph (b) is also catalyzed because it passes through an alternative pathway with a lower activation energy barrier.
Q21.4.27
Write a balanced equation for each of the following nuclear reactions:
- bismuth-212 decays into polonium-212
- beryllium-8 and a positron are produced by the decay of an unstable nucleus
- neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
- strontium-90 decays into yttrium-90
Solution Q.21.4.27
1. The first step in the process of writing these equations is determining the number of protons in each of these elements. Referring to the periodic table, Bi has 83 protons and Po has 84. The equation looks like this thus far: \(\ce{^{212}_{83}Bi}\rightarrow \ce{^{212}_{84}Po} + \ce{^{?}_{?}?}\)
We need to balance this equation to determine what ? is. Both Bi and Po have a mass number of 212, so the ? will have a mass number of zero. This means that ? is not an alpha particle, since it has a mass number of 4. Bi has the atomic number 83, while Po has the atomic number 84; to have both sides equal 83, the ? particle must have a -1 in that spot so that 84-1=83. The particle that matches these numbers is the electron beta particle, \(\ce{^{0}_{-1}e}\).
\(\ce{^{212}_{83}Bi}\rightarrow \ce{^{212}_{84}Po} + \ce{^{0}_{-1}e}\)
2. Be has 4 protons, and a positron has the formula \(\ce{^{0}_{+1}e}\). This is what we know about the reaction so far: \(\ce{^{?}_{?}?}\rightarrow \ce{^{8}_{4}Be} + \ce{^{0}_{+1}e}\)
Again we need to use some nuclear accounting to find out what decays into these products. The mass number of the element is going to be 8 because (mass # of Be)+(mass # of positron)=8. The atomic number is 4+1=5. Referencing the periodic table, the element with 5 protons is boron, so our missing element is \(\ce{^{8}_{5}B}\).
\(\ce{^{8}_{5}B}\rightarrow \ce{^{8}_{4}Be} + \ce{^{0}_{+1}e}\)
3. Finding the proton numbers from the periodic table, the first reaction looks like this: \(\ce{^{238}_{92}U} + \ce{^{1}_{0}n}\rightarrow \ce{^{239}_{93}Np}\)
However, this is not balanced. The neutron balances the mass numbers (238+1=239), but Np has an extra proton. Thus we need to subtract one proton from the right side of the equation by adding the beta particle \(\ce{^{0}_{-1}e}\).
\(\ce{^{238}_{92}U} + \ce{^{1}_{0}n}\rightarrow \ce{^{239}_{93}Np} + \ce{^{0}_{-1}e}\)
For the second part of this question we turn Np-239 into Pu-239. Looking at the periodic table, Np has 93 protons and Pu has 94, so the reaction so far is: \(\ce{^{239}_{93}Np}\rightarrow \ce{^{239}_{94}Pu} + \ce{^{?}_{?}?}\)
The mass number does not change, but we need to balance the proton numbers: 93=94+x. The ? particle has a proton number of -1, so this is once again an electron beta particle.
\(\ce{^{239}_{93}Np}\rightarrow \ce{^{239}_{94}Pu} + \ce{^{0}_{-1}e}\)
4. Referring to the periodic table, Sr has 38 protons and Y has 39. The reaction so far is: \(\ce{^{90}_{38}Sr}\rightarrow \ce{^{90}_{39}Y} + \ce{^{?}_{?}?}\)
Balancing this equation, one discovers that our ? particle has a mass of 0 and an atomic number of -1, so this is an electron beta particle.
\(\ce{^{90}_{38}Sr}\rightarrow \ce{^{90}_{39}Y} + \ce{^{0}_{-1}e}\)
Q20.3.13
For each galvanic cell represented by these cell diagrams, determine the spontaneous half-reactions and the overall reaction. Indicate which reaction occurs at the anode and which occurs at the cathode.
- Zn(s)∣Zn2+(aq) ∥ H+(aq)∣H2(g), Pt(s)
- Ag(s)∣AgCl(s)∣Cl−(aq) ∥ H+(aq)∣H2(g)∣Pt(s)
- Pt(s)∣H2(g)∣H+(aq) ∥ Fe2+(aq), Fe3+(aq)∣Pt(s)
Solution Q20.3.13
A cell diagram has the components of the oxidation reaction on the left side of the double lines and the reduction reaction on the right side. The electrodes are at the very left and the very right, and these can be inert; C and Pt are typically inert, and you do not include these in the reactions. Oxidation always happens at the anode, and reduction occurs at the cathode. Typically the reactant of the half-reaction is to the left of the product.
- reduction (cathode): 2H+(aq) + 2e− → H2(aq)
As said before, reduction is the right half of the cell and always at the cathode. Pt is an inert electrode. H+ is the reactant, since it is to the left of H2, the product. H2 has two hydrogen molecules, so you must give H+ a coefficient of 2 to balance this equation. To turn one H+ into Ho takes one electron, so performing this process twice requires two electrons.
oxidation (anode): Zn(s) → Zn2+(aq) + 2e−
The steps for this equation are about the same. It should be noted that Zno must lose two electrons to become Zn2+, and thus the electrons are on the right side of the equation.
overall: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(aq)
The amount of electrons lost and gained in the half-reactions is the same; H+ gains the two electrons that Zn loses. Thus these two equations can be combined without any multiplication of coefficients.
The process should be the same for the rest of the questions. The only unusual cell is the third one, where the reduction of Fe should happen in the opposite order, with Fe3+ turning into Fe2+.
- reduction (cathode): AgCl(s) + e− → Ag(s) + Cl−(aq)
oxidation (anode): H2(g) → 2H+(aq) + 2e−
overall: AgCl(s) + H2(g) → 2H+(aq) + Ag(s) + Cl−(aq)
- reduction (cathode): Fe3+(aq) + e− → Fe2+(aq)
oxidation (anode): H2(g) → 2H+(aq) + 2e−
overall: 2Fe3+(aq) + H2(g) → 2H+(aq) + 2Fe2+(aq)
(this question had answers but no explanations)
Q20.5.26
Ideally, any half-reaction with E° > 1.23 V will oxidize water as a result of the half-reaction O2(g) + 4H+(aq) + 4e− → 2H2O(l).
- Will FeO42− oxidize water if the half-reaction for the reduction of Fe(VI) → Fe(III) is FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O; E° = 1.9 V?
- What is the highest pH at which this reaction will proceed spontaneously if [Fe3+] = [FeO42−] = 1.0 M and PO2= 1.0 atm?
Solution Q20.5.26
1. Since the FeO42- reaction, with a Eo of 1.9 V, satisfies the requirement that E° > 1.23 V, FeO42- will oxidize water.
2. The first step of determining this is to write the full balanced equation. It should first be noted that the O2 equation needs to be an oxidation reaction, whereas right now it shows reduction. Turning it around, it looks like this: 2H2O(l) →O2(g) + 4H+(aq) + 4e−. However, we cannot combine the equations yet because the electrons are not balanced; O2 loses 4 electrons, while FeO42- only gains three. Thus we must multiply the O2 equation by three and the FeO42- equation by four.
4FeO42−(aq) + 32H+(aq) + 12e− + 6H2O(l)→ 4Fe3+(aq) + 16H2O(l) + 3O2(g) + 12H+(aq) + 12 e-
The electrons will cancel, but so will everything else in red; some simple subtraction gives us the final full equation:
4FeO42−(aq) + 20H+(aq) +→ 4Fe3+(aq) + 10H2O(l) + 3O2(g)
For determining the highest pH we will use the following equation: $$E_{cell} = E_{cell}^o - \dfrac{.0592 \, V}{n} \ln Q$$
We need to determine Eocell, Q, and n. Eocell is determined by subtracting the cathode Eo from the anode Eo. The cathode is where the reduction reaction happens, which in this case is the FeO4- reaction, and the anode is where oxidation happens, which is the O2 reaction. So Eocell = 1.9-1.23 = .67 V.
n is the number of electrons transferred; looking back at when we balanced our equation, this equals 12.
Q is the reaction quotient, equal to the concentration of the products over the concentration of the reactants with their coefficients as exponents. Working with the numbers given us ([Fe3+] = [FeO42−] = 1.0 M and PO2= 1.0 atm), we can create this equation: $$Q = \dfrac{[\ce{O_2}]^3[\ce{Fe^{3+}}]^4}{[\ce{H^+}]^{20}[\ce{FeO_4^{2-}}]^4}$$ $$Q = \dfrac{[1 M]^3[1 M]^4}{[\ce{H^+}]^{20}[\ce{1 atm}]^4} = \dfrac{1}{[\ce{H^+}]^{20}}$$
The entire point of this problem is to solve for [H+] when Ecell=0, determining what the lowest concentration of H+ can be with the reaction still being spontaneous, Ecell being positive. So we can plug in the values that we have and solve for [H+]: $$0 \lt .67 - \dfrac{.0592}{12} \ln \dfrac{1}{[\ce{H^+}]^{20}}$$ $$-.67 \lt - \dfrac{.0592}{12} \ln \dfrac{1}{[\ce{H^+}]^{20}}$$ $$-.67\left(\dfrac{12}{.0592}\right) \lt -\ln \dfrac{1}{[\ce{H^+}]^{20}}$$
Using a few logarithm laws, we can manipulate the natural log to make solving for H+ easier: $$-.67\left(\dfrac{12}{.0592}\right) \lt \ln {[\ce{H^+}]^{20}}$$ $$-135.811 \lt 20\ln {[\ce{H^+}]}$$ $$e^\left({\dfrac{-135.811}{20}}\right) \lt [\ce{H^+}]$$ $$[\ce{H^+}] \gt .0011243$$
This equation proves, then, that the pH needs to be lower than -log(.0011243)=2.95 for this reaction to be spontaneous under the given conditions.
(this question originally had no answer)

