Extra Credit 8
- Page ID
- 83287
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Edited by Olivia Walsh
Q17.1.7
Balance the following terms in basic solution:
\(\\\mathrm{a.}\,\mathrm{SO_3^{2-}} (aq)+\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{SO_4^{2-}}(aq)+\mathrm{Cu(OH)}(s)\\\mathrm{b.}\,\mathrm{O_2}(g)+\mathrm{Mn(OH)_2}(s)\longrightarrow \mathrm{MnO_2}(s)\\\mathrm{c.}\,\mathrm{NO_3^-}(aq)+\mathrm{H_2}(g)\longrightarrow \mathrm{NO}(g)\\\mathrm{d.}\,\mathrm{Al}(s)+\mathrm{CrO_4^{2-}}(aq)\longrightarrow \mathrm{Al(OH)_3}(s)+\mathrm{Cr(OH)_4^-}(aq)\)
A17.1.7
a.\(\mathrm{\,SO_3^{2-}} (aq)+\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{SO_4^{2-}}(aq)+\mathrm{Cu(OH)}(s)\)
Step 1. Separate into oxidation/reduction half reactions.
$$\\\mathrm{SO_3^{2-}}(aq)\longrightarrow \mathrm{SO_4^{2-}}(aq)$$
This is the oxidation because Sulfur is increasing in charge to balance out the new oxygens
$$\\\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{Cu(OH)}(s)$$
This is the reduction half because the copper is becoming more negative as it balances the charge of fewer OH molecules.
Step 2. First, balance the elements that are not \(\mathrm{H}\) or \(\mathrm{O}\). In this case, there are not any elements other than \(\mathrm{H}\) or \(\mathrm{O}\) that needs to be balanced. Balance \(\mathrm{O}\) first by adding \(\mathrm{H_2O}\) then balance \(\mathrm{H}\) by adding \(\mathrm{H^+}\)
$$\\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)\longrightarrow \mathrm{SO_4^{2-}}(aq)+ \mathrm{2H^+}$$
\(\mathrm{H_2O}\) added to the left side. \(\mathrm{2H^+}\) added to the right to balance the \(\mathrm{H}\) from \(\mathrm{H_2O}\).
Do the same for the other half-reaction: $$\\\mathrm{H^+}+\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{Cu(OH)}(s)+\mathrm{H_2O}$$
\(\mathrm{H_2O}\) added to the right side then \(\mathrm{H^+}\) added to the left side.
Step 3. Balance the charges by adding the appropriate amount of electrons.
$$ \\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)\longrightarrow \mathrm{SO_4^{2-}}(aq)+ \mathrm{2H^+}+\mathrm{2e^-}$$
Left: \(\mathrm{H_2O}\) has a neutral charge, \(\mathrm{SO_3^{2-}}\) has a -2 charge = -2 overall charge
Right: \(\mathrm{2H^+}\) has a +2 charge, \(\mathrm{SO_4^{2-}}\) has a -2 charge= 0 overall charge
Add \(\mathrm{2e^-}\) to the right side in order to balance charge of the equation.
$$\\\mathrm{e^-}+\mathrm{H^+}+\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{Cu(OH)}(s)+\mathrm{H_2O}$$
Left: \(\mathrm{H^+}\) has a +1 charge, \(\mathrm{Cu(OH)_2}\) has a neutral charge = +1 overall
Right: \(\mathrm{H_2O}\) has a neutral charge, \(\mathrm{Cu(OH)}\) has a neutral charge = 0 overall
Add \(\mathrm{e^-}\) to the left side in order to balance the charge of the equation.
Step 4. Amount of electrons in between the half reactions must be balanced by scaling the half reactions.
$$\\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)\longrightarrow \mathrm{SO_4^{2-}}(aq)+ \mathrm{2H^+}+\mathrm{2e^-}$$
This half reaction can be left unmodified.
$$\\\mathrm{(2)}*[\mathrm{e^-}+\mathrm{H^+}+\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{Cu(OH)}(s)+\mathrm{H_2O}]\leadsto\mathrm{2}\mathrm{e^-}+\mathrm{2}\mathrm{H^+}+\mathrm{2}\mathrm{Cu(OH)_2}(aq)\longrightarrow \mathrm{2}\mathrm{Cu(OH)}(s)+\mathrm{2}\mathrm{H_2O}$$
This half reaction is scaled by 2, so the number of electrons will be equal to the number of electrons in the other half reaction.
Step 5. Combine half reactions and cancel electrons.
$$\\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)+\mathrm{2e^-}+\mathrm{2H^+}+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2H^+}+\mathrm{2e^-}+ \mathrm{2Cu(OH)}(s)+\mathrm{2H_2O}$$
$$\\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)+\mathrm{2H^+}+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2H^+}+ \mathrm{2Cu(OH)}(s)+\mathrm{2H_2O}$$
Step 6. Add appropriate amount of \(\mathrm{OH^-}\) to balance \(\mathrm{H^+}\); combine to form \(\mathrm{H_2O}\).
$$\\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)+\mathrm{2H^+}+\mathrm{2OH^-}+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2H^+}+\mathrm{2OH^-}+ \mathrm{2Cu(OH)}(s)+\mathrm{2H_2O}$$
$$\\\mathrm{H_2O}+\mathrm{SO_3^{2-}}(aq)+\mathrm{2H_2O}+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2H_2O}+ \mathrm{2Cu(OH)}(s)+\mathrm{2H_2O}$$
Step 7. Cancel common terms.
$$\\\mathrm{3H_2O}+\mathrm{SO_3^{2-}}(aq)+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2Cu(OH)}(s)+\mathrm{4H_2O}$$
Total of 3 \(\mathrm{H_2O}\) on left side and 4 \(\mathrm{H_2O}\).
$$\\\mathrm{SO_3^{2-}}(aq)+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2Cu(OH)}$$
After cancellation, only 1 \(\mathrm{H_2O}\) remains in the right side.
Balanced equation:
$$\\\mathrm{SO_3^{2-}}(aq)+\mathrm{2Cu(OH)_2}(aq)\longrightarrow\mathrm{SO_4^{2-}}(aq)+ \mathrm{2Cu(OH)}(s)+\mathrm{H_2O}(l)$$
b. \(\\\mathrm{O_2}(g)+\mathrm{Mn(OH)_2}(s)\longrightarrow \mathrm{MnO_2}(s)\)
Step 1. Separate into reduction/oxidation half reactions.
$$\mathrm{Mn(OH)_2}(s)\longrightarrow\mathrm{MnO_2}(s)$$
$$\mathrm{O_2}(g)\longrightarrow\mathrm{H_2O}(l)$$
Looking at the oxidation states, \(\mathrm{Mn}\) goes from +4 in \(\mathrm{(Mn(OH)_2}\) to +2 in \(\mathrm{MnO_2}\). This means that \(\mathrm{(Mn(OH)_2}\) is oxidized since it loses electrons. Therefore, we can assume that the oxygen half reaction is reduction. When water is reduced it becomes water, so we can write the half reaction as above.
Step 2. Balance oxygen with water and hydrogen with protons since there are no other elements that needs to be balanced.
$$\mathrm{Mn(OH)_2}(s)\longrightarrow\mathrm{MnO_2}(s)+\mathrm{2H^+}(aq)$$
$$\mathrm{4H^+}(aq)+\mathrm{O_2}(g)\longrightarrow\mathrm{H_2O}(l)+\mathrm{H_2O}(l)$$
Step 3. Balance charges with electrons
$$\mathrm{Mn(OH)_2}(s)\longrightarrow\mathrm{MnO_2}(s)+\mathrm{2H^+}(aq)+\mathrm{2e^-}$$
\(\mathrm{(Mn(OH)_2}\) is neutral, so the left side has an overall 0 charge. \(\mathrm{MnO_2}\) has a neutral charge, \(\mathrm{2H^+}\) has a +2 charge so the right side has an overall +2 charge. To balance, add 2 electrons to the right side.
$$\mathrm{4H^+}(aq)+\mathrm{4e^-}+\mathrm{O_2}(g)\longrightarrow\mathrm{2H_2O}(l)$$
\(\mathrm{O_2}\) has a neutral charge, \(\mathrm{4H^+}\) has a +4 charge, so left has a total +4 charge. The right side has a 0 charge because of the neutral water molecules. To balance, add 4 electrons to the left side.
Step 4. Scale the oxidation half reaction by 2 to balance the electrons between both reactions.
$$\mathrm{2Mn(OH)_2}(s)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{4H^+}(aq)+\mathrm{4e^-}$$
$$\mathrm{4H^+}(aq)+\mathrm{4e^-}+\mathrm{O_2}(g)\longrightarrow\mathrm{2H_2O}(l)$$
Step 5. Now that the electrons are balance, we can cancel them.
$$\mathrm{2Mn(OH)_2}(s)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{4H^+}(aq)$$
$$\mathrm{4H^+}(aq)+\mathrm{O_2}(g)\longrightarrow\mathrm{2H_2O}(l)$$
Step 6. Combine the half reactions
$$\mathrm{2Mn(OH)_2}(s)+\mathrm{4H^+}(aq)+\mathrm{O_2}(g)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{4H^+}(aq)+\mathrm{2H_2O}(l)$$
Step 7. Add \(\,\mathrm{OH^-}\) to balance \(\mathrm{H^+}\). These combine to form water.
$$\mathrm{2Mn(OH)_2}(s)+\mathrm{4H^+}(aq)+\mathrm{4OH^-}(aq)+\mathrm{O_2}(g)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{4H^+}(aq)+\mathrm{4OH^-}(aq)+\mathrm{2H_2O}(l)$$
$$\mathrm{2Mn(OH)_2}(s)+\mathrm{4H_2O}(l)+\mathrm{O_2}(g)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{4H_2O}(l)+\mathrm{2H_2O}(l)$$
Step 8. Cancel common terms, in this case, cancel 4 water molecules from both sides.
$$\mathrm{2Mn(OH)_2}(s)+\mathrm{O_2}(g)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{2H_2O}(l)$$
Balanced equation:
$$\mathrm{2Mn(OH)_2}(s)+\mathrm{O_2}(g)\longrightarrow\mathrm{2MnO_2}(s)+\mathrm{2H_2O}(l)$$
c.\(\mathrm{NO_3^-}(aq)+\mathrm{H_2}(g)\longrightarrow \mathrm{NO}(g)\)
Step 1. Separate equations into half reactions
$$\mathrm{NO_3^-}(aq)\longrightarrow\mathrm{NO}(g)$$
$$\mathrm{H_2}(g)\longrightarrow\mathrm{H_2O}$$
We know that the \(\mathrm{NO_3^-}\) will be reduced because nitrogen's oxidation state goes from +5 to +2 in \(\mathrm{NO}\). It gains electrons, while \(\mathrm{H_2}\) loses electrons and is converted into water.
Step 2. Since there nitrogen is already balanced, balance oxygen(with water molecules) first and then balance hydrogen(with protons)
$$\mathrm{4H^+}(aq)+\mathrm{NO_3^-}(aq)\longrightarrow\mathrm{NO}(g)+\mathrm{2H_2O}(l)$$
$$\mathrm{H_2}(g)+\mathrm{H_2O}(l)\longrightarrow\mathrm{H_2O}(l)+\mathrm{4H^+}(aq)$$
Step 3. Balance the charges of each half reaction by adding electrons
$$\mathrm{3e^-}+\mathrm{4H^+}(aq)+\mathrm{NO_3^-}(aq)\longrightarrow\mathrm{NO}(g)+\mathrm{2H_2O}(l)$$
The left side has an overall 3+ charge(4+ from protons, and -1 from \(\mathrm{NO_3^-}\) ). The right side has an overall 0 charge since \(\mathrm{NO}\) and water are neutral molecules. Add 3 electrons to the left to balance.
$$\mathrm{H_2}(g)+\mathrm{H_2O}(l)\longrightarrow\mathrm{H_2^O}(l)+\mathrm{2H^+}(aq)+\mathrm{2e^-}$$
The left side has an overall 0 charge because \(\mathrm{H_2}\) is in its elemental form and water is neutral. The right side has a +2 overall charge because of the 2 protons. Add 2 electrons to the right side to balance.
Step 4. Scale the reactions to balance the electrons between both half reactions. Multiply the reduction reaction by 2 and the oxidation reaction by 3.
$$\mathrm{6e^-}+\mathrm{8H^+}(aq)+\mathrm{2NO_3^-}(aq)\longrightarrow\mathrm{2NO}(g)+\mathrm{4H_2O}(l)$$
$$\mathrm{3H_2}(g)+\mathrm{3H_2O}(l)\longrightarrow\mathrm{3H_2O}(l)+\mathrm{6H^+}(aq)+\mathrm{6e^-}$$
Step 5. Cancel electrons
$$\mathrm{8H^+}(aq)+\mathrm{2NO_3^-}(aq)\longrightarrow\mathrm{2NO}(g)+\mathrm{4H_2O}(l)$$
$$\mathrm{3H_2}(g)+\mathrm{3H_2O}(l)\longrightarrow\mathrm{3H_2O}(l)+\mathrm{6H^+}(aq)$$
Step 6. Add \(\,\mathrm{OH^-}\) to balance \(\mathrm{H^+}\).
Add \(\,\mathrm{8OH^-}\) to the right and left sides of the reduction reaction equation.
$$\mathrm{8H^+}(aq)+\mathrm{8OH^-}(aq)+\mathrm{2NO_3^-}(aq)\longrightarrow\mathrm{2NO}(g)+\mathrm{4H_2O}(l)+\mathrm{8OH^-}(aq)$$
Combine the protons and hydroxide ions to form water
$$\mathrm{8H_2O}(l)+\mathrm{2NO_3^-}(aq)\longrightarrow\mathrm{2NO}(g)+\mathrm{4H_2O}(l)+\mathrm{8OH^-}(aq)$$
For the oxidation reaction equation, add \(\,\mathrm{6OH^-}\) to both sides of the equation.
$$\mathrm{6OH^-}(aq)+\mathrm{3H_2}(g)+\mathrm{3H_2O}(l)\longrightarrow\mathrm{3H_2O}(l)+\mathrm{6H^+}(aq)+\mathrm{6OH^-}(aq)$$
Combine the protons and hydroxide ions to form water
$$\mathrm{6OH^-}(aq)+\mathrm{3H_2}(g)+\mathrm{3H_2O}(l)\longrightarrow\mathrm{3H_2O}(l)+\mathrm{6H_2O}(l)$$
Step 7. Combine the half reactions into one mega equation
$$\mathrm{11H_2O}(l)+\mathrm{2NO_3^-}(aq)+\mathrm{6OH^-}(aq)+\mathrm{3H_2}(g)\longrightarrow\mathrm{2NO}(g)+\mathrm{13H_2O}(l)+\mathrm{8OH^-}(aq)$$
Step 8. Cancel common terms
$$\mathrm{2NO_3^-}(aq)+\mathrm{3H_2}(g)\longrightarrow\mathrm{2NO}(g)+\mathrm{2H_2O}(l)+\mathrm{2OH^-}(aq)$$
Balanced equation:
$$\mathrm{2NO_3^-}(aq)+\mathrm{3H_2}(g)\longrightarrow\mathrm{2NO}(g)+\mathrm{2H_2O}(l)+\mathrm{2OH^-}(aq)$$
d. \(\\\mathrm{Al}(s)+\mathrm{CrO_4^{2-}}(aq)\longrightarrow \mathrm{Al(OH)_3}(s)+\mathrm{Cr(OH)_4^-}(aq)\)
Step 1. Separate into half reactions. Here, aluminum is oxidized since its oxidation number goes from 0 to +3 (loses electrons). Chromate is reduced; chromium's oxidation state goes from +6 to +3 (gains electrons).
$$\mathrm{Al}(s)\longrightarrow\mathrm{Al(OH)_3}(s)$$
$$\mathrm{CrO_4^{2-}}(aq)\longrightarrow\mathrm{Cr(OH)_4^-}(aq)$$
Step 2. Both chromium and aluminum are already balanced. The next step is to balance oxygen, then hydrogen with water and protons, respectively.
$$\mathrm{Al}(s)+\mathrm{3H_2O}(l)\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{3H^+}(aq)$$
$$\mathrm{CrO_4^{2-}}(aq)+\mathrm{4H^+}(aq)\longrightarrow\mathrm{Cr(OH)_4^-}(aq)$$
Step 3. Balance charges with electrons
The left side has 0 charge because aluminum is in its elemental form and water is a neutral molecule. The right side has a +3 charge because of the 3 protons and \(\mathrm{Al(OH)_3}\) has 0 charge. So, add 3 electrons to the right side to balance.
$$\mathrm{Al}(s)+\mathrm{3H_2O}(l)\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{3H^+}(aq)+\mathrm{3e^-}$$
The left side has an overall +2 charge since chromate has a -2 charge and the +4 charge from the protons. The right side has a -1 charge because \(\mathrm{Cr(OH)_4^-}\) has -1 charge. To balance, add 3 electrons to the left side.
$$\mathrm{CrO_4^{2-}}(aq)+\mathrm{4H^+}(aq)+\mathrm{3e^-}\longrightarrow\mathrm{Cr(OH)_4^-}(aq)$$
Step 4. Balance the protons by adding the appropriate amount hydroxide ions to both side of each equation.
$$\mathrm{Al}(s)+\mathrm{3H_2O}(l)+\mathrm{3OH^-}(aq)\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{3H^+}(aq)+\mathrm{3OH^-}(aq)+\mathrm{3e^-}$$
$$\mathrm{CrO_4^{2-}}(aq)+\mathrm{4H^+}(aq)+\mathrm{4OH^-}(aq)+\mathrm{3e^-}\longrightarrow\mathrm{Cr(OH)_4^-}(aq)+\mathrm{4OH^-}(aq)$$
Step 5. Combine the equations together. Also combine the protons and hydroxide to form water.
$$\mathrm{Al}(s)+\mathrm{3H_2O}(l)+\mathrm{3OH^-}(aq)+\mathrm{CrO_4^{2-}}(aq)+\mathrm{4H^+}(aq)+\mathrm{4OH^-}(aq)+\mathrm{3e^-}\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{3H^+}(aq)+\mathrm{3OH^-}(aq)+\mathrm{3e^-}+\mathrm{Cr(OH)_4^-}(aq)+\mathrm{4OH^-}(aq)$$
$$\mathrm{Al}(s)+\mathrm{3H_2O}(l)+\mathrm{3OH^-}(aq)+\mathrm{CrO_4^{2-}}(aq)+\mathrm{4H_2O}(l)+\mathrm{3e^-}\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{3H_2O}(l)+\mathrm{3e^-}+\mathrm{Cr(OH)_4^-}(aq)+\mathrm{4OH^-}(aq)$$
As seen above, the protons and hydroxide form water.
Below, all the water molecules that are on the same side are combined.
$$\mathrm{Al}(s)+\mathrm{7H_2O}(l)+\mathrm{3OH^-}(aq)+\mathrm{CrO_4^{2-}}(aq)+\mathrm{3e^-}\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{3H_2O}(l)+\mathrm{3e^-}+\mathrm{Cr(OH)_4^-}(aq)+\mathrm{4OH^-}(aq)$$
Step 6. Finally, cancel common terms
$$\mathrm{Al}(s)+\mathrm{4H_2O}(l)+\mathrm{CrO_4^{2-}}(aq)\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{Cr(OH)_4^-}(aq)+\mathrm{OH^-}(aq)$$
Balanced equation:
$$\mathrm{Al}(s)+\mathrm{4H_2O}(l)+\mathrm{CrO_4^{2-}}(aq)\longrightarrow\mathrm{Al(OH)_3}(s)+\mathrm{Cr(OH)_4^-}(aq)+\mathrm{OH^-}(aq)$$
Very clear steps all the way through, I caught no mistakes.
Q19.1.6
Which of the following is the strongest oxidizing agent: \(\mathrm{VO_4^{3-}},\, \mathrm{CrO_4^{2-}},\,\mathrm{MnO_4^-}\)?
A19.1.6
Oxidizing agents oxidize other substances. In other words, they gain electrons or become reduced. These agents should be in their highest oxidation state. In order to determine, the strength of the compounds above as oxidizing agents, determine the oxidation numbers of each constituent elements.
\(\\\mathrm{VO_4^{3-}}\)
We know that \(\mathrm{O}\) has a -2 oxidation state and the overall charge of the ion is -3. We just need to determine Vanadate's oxidation number in this compound.
\(\\\mathrm{V} + \mathrm{-2(4)} = \mathrm{-3}\)
\(\\\mathrm{V} = \mathrm{+5}\)
Vanadate has an oxidation number of +5, which is its highest possible oxidation state.
\(\\\mathrm{CrO_4^{2-}}\)
Like in the previous calculation, \(\mathrm{O}\) has a -2 oxidation state. The overall charge is -2. So calculate for chromium.
\(\\\mathrm{Cr} + \mathrm{-2(4)} = \mathrm{-2}\)
\(\\\mathrm{Cr} = \mathrm{+6}\)
Chromium is in its highest possible oxidation state of +6 in this compound.
\(\\\mathrm{MnO_4^-}\)
\(\mathrm{O}\) has a -2 oxidation state and the overall charge is -1.
\(\\\mathrm{Mn} + \mathrm{-2(4)} = \mathrm{-1}\)
\(\\\mathrm{Mn} = \mathrm{+7}\)
Manganese is also in its highest oxidation state, +7.
An oxidizing agent has to be able to gain electrons which, in turn, reduces its oxidation state. Here manganese has the greatest oxidation state which allows it to experience a greater decrease in its oxidation state if needed, meaning it can gain the most electrons. So among the three compounds, \(\mathrm{MnO_4^-}\) is the strongest oxidizing agent.
Clear and thorough explanation.
Q19.2.8
Specify whether the following complexes have isomers.
- tetrahedral \(\mathrm{[Ni(CO)_2(Cl)_2]}\)
- trigonal bipyramidal \(\mathrm{[Mn(CO)_4(NO)]}\)
- \(\mathrm{[Pt(en)_{2}Cl_2]Cl_2}\)
A19.2.8
Isomers are compounds that have the same number of atoms, but have different structures. Structural isomers (linkage, ionization, coordination) and stereoisomers (geometric and optical) can occur with several compounds.
1. tetrahedral \(\mathrm{[Ni(CO)_2(Cl)_2]}\)
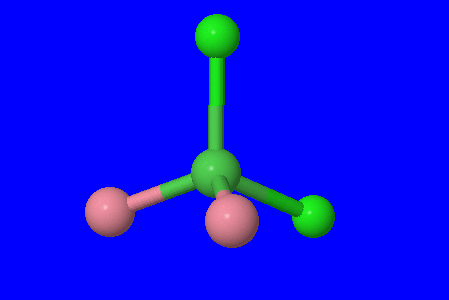
(Fig 1.) In this model, nickel is the dark green central atom, carbonyl ligands are the pink atom, and chloro ligands are the light green atoms.
Immediately, we can cancel out the possibility of linkage, ionization, and coordination isomers. There are no other coordination complexes for coordination isomerism, there is no ligand that can bond to the atom in more than one way for it to exhibit linkage isomerism, and there are no ions outside the coordination sphere for ionization isomerism.
This is a tetrahedral structure which immediately rules out any geometric isomers since they require 90° and/or 180° bond angles. Tetrahedral structures have 109.5° angles.
To confirm that the structure has no optical isomer, we must determine if there is a plane of symmetry. Structures that have no plane of symmetry are considered chiral and would have optical isomers.
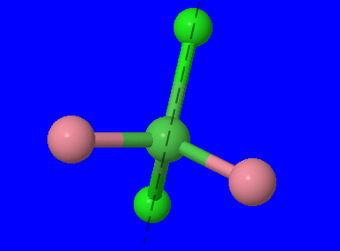
(Fig 2.) We can rotate the structure and find that there is indeed a plane of symmetry through the two chloro ligands and central atom and between the carbonyl ligands.
Since there is a plane of symmetry, we can conclude that there are no optical isomers.
Overall, there are no isomers that exist for this compound.
2. trigonal byprimidal \(\mathrm{[Mn(CO)_4(NO)]}\)
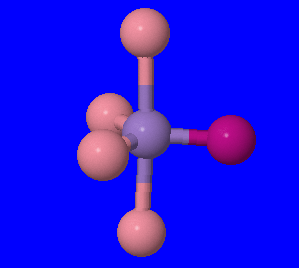
(Fig 3.) The central purple atom is manganese, the carbonyl ligands are the pink atoms, and the nitrosyl ligand is the fuschia atom.
There are no ions, other coordination complex, and ambidentate ligands. Therefore, no structural isomers exist for this structure.
Geometric isomers do not exist for this compound because there is only one nitrosyl ligand.
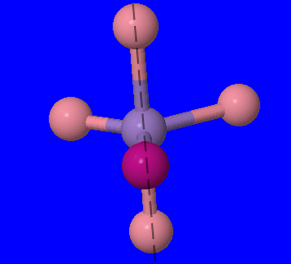
(Fig 4.) Dashed line bisects molecule and shows plane of symmetry. The molecule is rotated in this image.
In the image above, after the structure has been rotated, we can see that there is a plane of symmetry. Thus, there are no optical isomers.
No isomers (the ones mentioned above) exist for this compound.
3. \(\mathrm{[Pt(en)_{2}Cl_2]Cl_2}\)
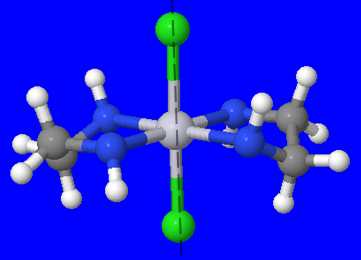
(Fig 5.) The green atoms are the chloro ligands, the the central atom is platinum, and the grey/blue atoms are ethyldiamine ligands.
Coordination isomerism cannot exist for this complex because there are no other complexes. There are no linkage isomers because there are no ambidentate ligands. Ionization isomers cannot exist in this complex either, even though there is a neutral molecule outside the coordination sphere. If we exchange \(\mathrm{Cl_2}\) with one ethyldiamine molecule, There would be 5 ligands in the coordination sphere instead of 4. This difference in the ratio of metal atom to ligands means that an ionization isomer cannot exist.
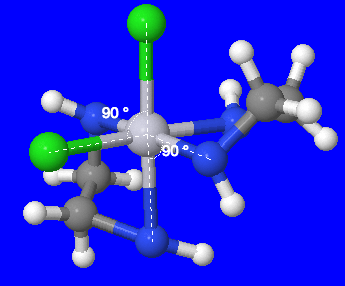
(Fig 6.) Here, one chloro ligand exchanged places with the ethyldiamine so that it can be at a 90° angle with the other chloro ligand.
The image above, shows the chloro and ethyldiamine ligands at a 90° angle with its other identical ligand. This is the cis isomer, while Fig. 5 shows the trans isomer.
Fig 5. shows that there is a plane of symmetry in the trans isomer. Therefore, that structure does not have an optical isomer. On the other hand, the cis isomer does not have a plane of symmetry and therefore has an optical isomer.
The diagrams are incredibly helpful, source included was good and each had a nice, clear explanation to help.
All figures above were created using VMK(Virtual Molecular Model Kit) at www.chemagic.org
Q12.3.21
The annual production of HNO3 in 2013 was 60 million metric tons. Most of that was prepared by the following sequence of reactions, each run in a separate reaction vessel.
a.\(\mathrm{4NH_3}(g) + \mathrm{5O_2}(g)\longrightarrow \mathrm{4NO}(g)+\mathrm{6H_2O}(g)\)
b.\(\mathrm{2NO}(g) + \mathrm{O_2}(g)\longrightarrow \mathrm{2NO_2}(g)\)
c.\(\mathrm{3NO_2}(g) + \mathrm{H_2O}(l)\longrightarrow \mathrm{2HNO_3}(aq)+\mathrm{NO}(g)\)
The first reaction is run by burning ammonia in air over a platinum catalyst. This reaction is fast. The reaction in equation (c) is also fast. The second reaction limits the rate at which nitric acid can be prepared from ammonia. If equation (b) is second order in NO and first order in O2, what is the rate of formation of NO2 when the oxygen concentration is 0.50 M and the nitric oxide concentration is 0.75 M? The rate constant for the reaction is 5.8 × 10−6 L2/mol2/s.
A12.3.21
We are given the information hat the second reaction (b) limits the rate at which nitric acid can be prepared from ammonia. From this, we can conclude that reaction (b) is the slow step and therefore determines the rate law of the reaction. Since the step is an elementary step, we can use the stoichiometric coefficients to determine the rate law (we are also told that NO is second order and O2 is first order). Remember that the rate law only includes the reactants.
We can write the rate law as
$$\\\mathrm{rate}=k\mathrm{[NO]^2[O_2]}$$
Now plug in the given concentration values. Note: the units for the rate constant, L2/mol2/s, can be written as M-2 \(\centerdot\)s-1
$$\\\mathrm{rate}=\dfrac{5.8\times10^{-6}}{M^2 \centerdot s}\mathrm{[0.50\,M]^2[0.75\,M]}$$
and solve to get
$$\\\mathrm{rate}=\mathrm{1.63125\times 10^{-6}\,M \centerdot s^{-1}}$$
We know that the reaction rate can be defined as the change in concentration over the change in time and would stay the same for any of the reactants or products. In other words, the rate of the consumption the reactants is equal to the rate of production of the products.
The rate of the formation of \(\mathrm{NO_2}\) can be given by
$$\\\mathrm{rate}=\dfrac{\Delta{[NO_2]}}{2\Delta{t}}$$
Rearrange the equation to solve for \(\dfrac{\Delta{[NO_2]}}{\Delta{t}}\)
$$\\{\dfrac{\Delta{[NO_2]}}{\Delta{t}}}=\mathrm{2\times rate}$$
Plug in the rate of the reaction
$$\\{\dfrac{\Delta{[NO_2]}}{\Delta{t}}}=\mathrm{(2)(1.63125\times 10^{-6}\,M \centerdot s^{-1})}$$
So, the rate of the formation of NO2 is \(3.2625\times 10^{-6}\,M \centerdot s^{-1}\)
Q12.7.1
Account for the increase in reaction rate brought about by a catalyst.
A12.7.1
Before a reaction can continue, it must reach a minimum amount of energy called the activation energy. Sometimes the activation energy of a reaction is very high which prevents the reaction from starting. Catalysts are substances that are added into the reaction in order to increase the rate of the reaction. Catalysts are not consumed in the process; they remain unchanged. These substances work by lowering the activation energy of a reaction or providing a different reaction mechanism. By lowering the activation energy, the reaction can continue at a much faster rate since the energy it would need to attain is lower.

The graph above shows the effect of a catalyst on a reaction. Without the catalyst, the activation energy is higher and there is only one transition state. With the catalyst, the activation energy is significantly lower and there are now four transition states. Note that \(\Delta{G}\) remains the same with or without the catalyst because the thermodynamics of a reaction is not affected by catalysts.
Q21.4.24
A \(\mathrm{^7_4}\mathrm{Be}\) atom (mass = 7.0169 amu) decays into a \(\mathrm{^7_3}\mathrm{Li}\) atom (mass = 7.0160 amu) by electron capture. How much energy (in millions of electron volts, MeV) is produced by this reaction?
A21.4.24
Electron capture is when an electron in the inner shell is absorbed into the nucleus. This causes a series of events to occur: a proton is converted into a neutron, a neutrino is emitted, and an electron from the next shell falls into the inner shell which causes an x-ray to be emitted.
This certain reaction has the balanced equation:
$$\\\mathrm{^7_4Be} + \mathrm{\ce{^0_{-1}e}}\longrightarrow \mathrm{^7_3Li} + v_e$$
Step 1. Calculate the mass defect \(\Delta{m}\) = Mass of products - Mass of reactants (Note: neutrinos ( \(v_e\)) have no charge and have a very very low mass that it is almost massless, so we will not include it in the calculation.)
Mass of reactants: \(\mathrm{^7_4Be}\) is 7.0169 amu, \(\mathrm{\ce{^0_{-1}e}}\) is \(\mathrm{5.49\times 10^{-4}}\) amu
$$\\\mathrm{7.0169\,amu} + \mathrm{5.49\times 10^{-4}\,amu} = \mathrm{7.01745\,amu}$$
Mass of product: \(\mathrm{^7_3Li}\) is 7.0160 amu
$$\\\Delta{m}=\mathrm{7.01745\, amu} - \mathrm{7.0160\,amu}$$
$$\\\Delta{m}=\mathrm{0.001449\,amu}$$
Step 2. Convert amu into kg.
$$\\\mathrm{0.001449\,amu}\,\times\dfrac{1.66\times10^{-27}\,\mathrm{kg}}{\mathrm{1\,amu}}=\mathrm{2.4046\times10^{-30}\,kg}$$
Step 3. Plug values into famous equation: \(\mathrm{E=mc^2}\);\(\,\,\,\mathrm{c=2.998\times10^8} \,m\centerdot s^{-1}\)
$$\\\mathrm{E} = (\mathrm{2.4046\times10^{-30}\,kg})(\mathrm{2.998\times10^8\,m \centerdot s^{-1}})^2$$
$$\\\mathrm{E} = \mathrm{2.1613\times10^{-13}}\mathrm{\,\,\,\dfrac{kg \centerdot m^2}{s^2}} = \mathrm{2.1613\times10^{-13}J}$$
Step 4. Convert J into MeV
$$\\\mathrm{2.1613\times10^{-13}\,\mathrm{J}}\,\times\dfrac{1\,\mathrm{MeV}}{1.602\times10^{-13}\mathrm{J}}=\mathrm{1.3491\,MeV}$$
\(\mathrm{1.3491\,MeV}\) is produced by the reaction.
Clear, understandable explanation of the math. Saw no errors.
Q20.3.11
Sulfate is reduced to HS− in the presence of glucose, which is oxidized to bicarbonate. Write the two half-reactions corresponding to this process. What is the equation for the overall reaction?
A20.3.11
Sulfate is reduced to \(\mathrm{HS^-}\). This is the reduction half reaction which is written as:
$$\\\mathrm{SO_4^{2-}}(aq)\longrightarrow\mathrm{HS^-}(aq)$$
Balance the half reaction
1. Balance oxygen by adding 4 \(\mathrm{H_2O}\) to the right side
2. Balance hydrogen by adding 9 \(\mathrm{H^+}\) to the left side.
3. Balance the charge by adding 8 electrons to the left side. ( Left: \(\mathrm{SO_4^{2-}}\) has -2 charge, \(\mathrm{9H^+}\) has a +9 charge = +7 charge, Right: \(\mathrm{HS^-}\) has a -1 charge, \(\mathrm{H_2O}\) has a neutral charge = -1 charge. Add 8 electrons to the left side to balance out. )
$$\\\mathrm{8e^-}+\mathrm{9H^+}(aq)+\mathrm{SO_4^{2-}}(aq)\longrightarrow\mathrm{HS^-}(aq)+\mathrm{4H_2O}(l)$$
Glucose is oxidized to bicarbonate. This is the oxidation half reaction which is written as:
$$\\\mathrm{C_6H_{12}O_6}(aq)\longrightarrow\mathrm{HCO_3^-}(g)$$
Balance the half reaction
1. Balance carbon first by adding the coefficient 6 to \(\mathrm{HCO_3^-}\) = \(\mathrm{6HCO_3^-}\)
2. Balance oxygen by adding 12 \(\mathrm{H_2O}\) to the left side.
3. Balance hydrogen by adding 30 \(\mathrm{H^+}\) to the right side.
4. Balance the charge by adding 24 electrons to the right side. ( Left: \(\mathrm{H_2O}\) has neutral charge, \(\mathrm{C_6H_{12}O_6}\) has neutral charge = 0 charge, Right: \(\mathrm{6HCO_3^-}\) has total of -6 charge \(\mathrm{30H^+}\) has total +30 charge = +24 charge. Add 24 electrons to the right side to balance out. )
$$\\\mathrm{12H_2O}(l)+\mathrm{C_6H_{12}O_6}(g)\longrightarrow\mathrm{6HCO_3^-}(g)+\mathrm{30H^+}(aq)+\mathrm{24e^-}$$
For the overall reaction, first, multiply the reduction half reaction by 3 to scale the equation in order to balance the electrons.
$$\\\mathrm{24e^-}+\mathrm{27H^+}(aq)+\mathrm{3SO_4^{2-}}(aq)\longrightarrow\mathrm{3HS^-}(aq)+\mathrm{12H_2O}(l)$$
Then combine the two half reactions and cancel common terms
$$\\\mathrm{24e^-}+\mathrm{27H^+}(aq)+\mathrm{3SO_4^{2-}}(aq)+\mathrm{12H_2O}+\mathrm{C_6H_{12}O_6}(aq)\longrightarrow\mathrm{3HS^-}(aq)+\mathrm{12H_2O}(l)+\mathrm{6HCO_3^-}(aq)+\mathrm{30H^+}(aq)+\mathrm{24e^-}$$
Overall:
$$\\\mathrm{3SO_4^{2-}}(aq)+\mathrm{C_6H_{12}O_6}(aq)\longrightarrow \mathrm{3HS^-}(aq)+\mathrm{6HCO_3^-}(aq)+\mathrm{3H^+}(aq)$$
Q20.5.23
For the reduction of oxygen to water, E° = 1.23 V. What is the potential for this half-reaction at pH 7.00? What is the potential in a 0.85 M solution of NaOH?
A20.5.23
In order to determine the potential at pH 7.00, it would be helpful to write the balanced half reaction.
Step 1. Balanced reaction
$$\\\mathrm{O_2}(g)\longrightarrow\mathrm{H_2O}(l)$$
Add \(\mathrm{H_2O}\) to the right side in order to balance the oxygen.
$$\\\mathrm{O_2}(g)\longrightarrow\mathrm{H_2O}(l)+\mathrm{H_2O}(l)$$
Then add \(\mathrm{H^+}\) to the left side to balance hydrogen
$$\\\mathrm{4H^+}(aq)+\mathrm{O_2}(g)\longrightarrow\mathrm{H_2O}(l)+\mathrm{H_2O}(l)$$
The total charge of the left side is +4 ( \(\mathrm{O_2}\) has a 0 charge, there are 4 protons with a total of +4 charge) , while the charge of the right side is 0 (water has a neutral charge). To balance the charges, add 4 electrons to the left side.
$$\\\mathrm{4e^-}+\mathrm{4H^+}(aq)+\mathrm{O_2}(g)\longrightarrow\mathrm{2H_2O}(l)$$
From this equation we can see the reactants and the products which would help us with calculating the reaction quotient, \(\mathit{Q}\), which we will plug in the Nernst equation.
Step 2. Find \(\mathit{Q}\).
$$\\\mathit{Q}=\mathrm{\dfrac{1}{P_{O_2}[H^+]^4}}$$
In this equation, the numerator (product) is has an activity of 1 because water is in its liquid state. The denominator is the activity of \(\mathrm{O_2}\) (given by the pressure of oxygen because it is a gas) and the activity (concentration) of the protons. Note: the concentration of the proton is raised to the power of 4 because of its coefficient.
$$\\\mathit{Q}=\mathrm{\dfrac{1}{1\,atm\,\,[1\times10^{-7}\, M]^4}}$$
Here, we plugged in values. Assuming, it is in standard conditions, the pressure of oxygen is 1 atm. Given that the pH is 7.00, we can calculate the \(\mathrm{H^+}\) concentration using \(\mathrm{[H^+]} = \mathrm{10^{-pH}}\).
Simply the equation to get:
$$\\\mathit{Q}=\mathrm{1\times10^{28}}$$
Now we plug in \(Q\), \(n\) (number of electrons), and the given \(E^o\) into the Nernst equation
$$E = E^o - \dfrac{0.0592\, V}{n} \log Q$$
$$E = 1.23\,V - \dfrac{0.0592\, V}{4} \log (1\times10^{28})$$
Simplify and get:
$$E = 1.23\,V- \dfrac{0.0592\, V}{4} (28)$$
$$E = 1.23\,V - 0.414\,V $$
$$E = 1.23\,V - 0.414\,V $$
$$E = 0.816\,V$$
The potential for this half reaction is 0.816 V at a pH of 7.00
In a 0.85 M NaOH solution:
In order to solve this problem, we must figure out the pH of the solution. Given that the concentration of NaOH is 0.85 M, the hydroxide ion concentration is also 0.85 M. Since we want the pH, we need to convert the concentration into pOH first.
To find the pOH, use \(\mathrm{pOH=-log[OH^-]}\)
$$\mathrm{pOH=-log[0.85]}$$
$$\mathrm{pOH=0.07058}$$
Now that we have pOH, we can determine pH by subtracting pOH from 14 (pOH+pH=14).
$$\mathrm{pH=14-0.0758}$$
$$\mathrm{pH=13.929}$$
Now, find the \(H^+\) concentration using the pH (use \(\mathrm{[H^+]} = \mathrm{10^{-pH}}\) ).
$$\mathrm{[H^+]} = \mathrm{10^{-13.929}}$$
$$\mathrm{[H^+]} = 1.1766\times10^{-14}$$
We can now plug in this value to find \(Q\).
$$\\\mathit{Q}=\mathrm{\dfrac{1}{1\,atm\,\,[1.1765\times10^{-14}\,M]^4}}$$
$$\\\mathit{Q}=5.1999\times10^{55}$$
Now plug this into the Nernst equation
$$E = 1.23\,V - \dfrac{0.0592\, V}{4} \log (5.1999\times10^{55})$$
$$E = 1.23\,V - \dfrac{0.0592\, V}{4} (55.716)$$
$$E = 1.23\,V - 0.8246\,V$$
$$E = 0.405\,V$$
In 0.85 M of NaOH, the cell potential is 0.405 V.

