Extra Credit 29
- Page ID
- 83261
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.4.2
For the ΔG° values given here, determine the standard cell potential for the cell.
- 12 kJ/mol, n = 3
- −45 kJ/mol, n = 1
Solution:
1.
\[\begin{align}
\Delta G&=-nFE^{o}_{cell}\\
12kJ\times 1000\dfrac{J}{kJ}&=-3mole\times96485\dfrac{Coulombus}{mole}\times E^{o}_{cell}\\
12000J&=-289455Coulombus\times E^{o}_{cell}\\
E^{o}_{cell}&=\dfrac{12000J}{-289455Coulombus}\\
E^{o}_{cell}&=-0.0415\dfrac{J}{Coulombus}\\
E^{o}_{cell}&=-0.0415V\\
\end{align}\]
2.
\[\begin{align}
\Delta G&=-nFE^{o}_{cell}\\
-45kJ\times 1000\dfrac{J}{kJ}&=-1mole\times96485\dfrac{Coulombus}{mole}\times E^{o}_{cell}\\
-45000J&=-96485Coulombus\times E^{o}_{cell}\\
E^{o}_{cell}&=\dfrac{45000J}{96485Coulombus}\\
E^{o}_{cell}&=0.466\dfrac{J}{Coulombus}\\
E^{o}_{cell}&=0.466V\\
\end{align}\]
Q12.1.2
Ozone decomposes to oxygen according to the equation \[2O_{3(g)}\rightarrow3O_{2(g)}\] . Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen.
Solution:
\[rate=-\dfrac{\Delta[O_{3(g)}]}{2\Delta t}=\dfrac{\Delta[O_{2(g)}]}{3\Delta t}\]
Q12.4.20
For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene (CH2=CH–CH=CH2)(CH2=CH–CH=CH2) has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
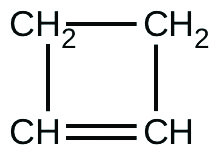
The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0 × 10−4 s−1 at 150 °C in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 °C with an initial pressure of 55 torr.
Solution:
The equation of the integrated rate law for a first order reaction is,
\(\begin{align}
ln\left(\dfrac{[A_{o}]}{[A]}\right)&=-kt\\
ln\left(\dfrac{[A]}{[A]_{o}}\right)&=kt\\
ln\left(\dfrac{p_{o}}{p_{t}}\right)&=kt\\
\end{align}\)
\(p_{o}\) is the initial partial pressure of the reactant (cyclobutene).
\(p_{t}\) is the concentration of the reactant (cyclobutene) left after 30.0 minutes.
k is the rate constant.
\([p_{o}]=55 torr\)
\(k=2.0\times 10^{-4} s^{-1}\)
\(t=30 minutes= 1800 seconds\)
Therefore,
\(\begin{align}
ln\left(\dfrac{55}{p_{t}}\right)&=2\times10^{-4}s^{-1}\times1800s\\
ln(55)-ln(p_{t})&=0.36\\
ln(p)&=3.647\\
p&=38.372 torr\\
\end{align}\)
The partial pressure of cyclobutene is 38.372 torr.
In order to find the concentration of cyclobutene, we can apply Henry's law,
\(p=K\times C\)
Also,
\(K=R\times T\)
Therefore,
\(\begin{align}
p&=R\times T\times C\\
38.35torr\times0.0013158 \dfrac {atm}{torr}&= 0.082L \dfrac {L\times atm}{mol\times K}\times 432K\times C\\
0.05046atm &=35.424\dfrac{L\times atm }{mol}\times C\\
C&=1.4245\times 10^{-3}\dfrac{mol}{L}\\
\end{align}\)
The concentration of cyclobutene after 30 mins is \(1.4245\times 10^{-3}\dfrac{mol}{L}\)
Q21.3.4
Complete each of the following equations:
a. \(\ce{^7_3Li}+\,?\rightarrow 2{^4_2He}\)
b. \(\ce{^{14}_6C}\rightarrow {^{14}_7N}+\,?\)
c. \(\ce{^{27}_{13}Al}+{^4_2He}\rightarrow\,?+{^1_0n}\)
d. \(\ce{^{250}_{96}Cm\rightarrow \,?+^{98}_{38}Sr}+4{^1_0n}\)
solution:
a. \(\ce{^7_3Li}+{^1_1n}\rightarrow 2{^4_2He}\)
b. \(\ce{^{14}_6C}\rightarrow {^{14}_7N}+{^0_{-1}e}\)
c. \(\ce{^{27}_{13}Al}+{^4_2He}\rightarrow {^{30}_{15}P}+{^1_0n}\)
d. \(\ce{^{250}_{96}Cm}\rightarrow {^{148}_{58}Ce}+{^{98}_{38}Sr}+4{^1_0n}\)
Q20.1.1
Identify the oxidation state of the atoms in the following compounds:
a. \(PCl_3\)
b. \(CO_3^{2-}\)
c. \(H_2S\)
d. \(S_8\)
e. \(SCl_2\)
f. \(Na_2SO_3\)
g. \(SO_4^{2-}\)
solution:
a. \(P=+3, Cl=-1\)
b. \(C=+4, O=-2\)
c. \(H=+1, S=-2\)
d. \(S=0\)
e. \(S+2, Cl=-1\)
f. \(Na=+1, S=+4, O=-2\)
g. \(S=+6, O=-2\)
Q20.4.19
Carbon is used to reduce iron ore to metallic iron. The overall reaction is as follows:
\[2Fe_2O_3\cdot xH_2O(s)+ 3C(s) \rightarrow 4Fe(l)+3CO_2(g)+xH_2O(g)\]
Write the two half-reactions for this overall reaction.
solution:
\[\begin{align}
12e^{-}+12H^{+}(aq)+2Fe_2O_3\cdot xH_2O(s)&\rightarrow4Fe(l)+(6+x)H_2O(g)\\
2H_2O(g)+3C(s) &\rightarrow CO_2(g)+4H^{+}(aq)+4e^{-}\\
\end{align}\]
Q20.9.4
Two solutions, one containing Fe(NO3)2·6H2O and the other containing the same molar concentration of Fe(NO3)3·6H2O, were electrolyzed under identical conditions. Which solution produced the most metal? Justify your answer.
solution:
Fe(NO3)2·6H2O produced more metal than Fe(NO3)3·6H2O.
Since Fe+2 in Fe(NO3)2·6H2O only needed two moles of electrons to produce one mole of iron metal while Fe+3 needed three moles, when they were electrolyzed under same condition, Fe(NO3)2·6H2O were able to produce more metal.
Q20.8.3
Why is it important for automobile manufacturers to apply paint to the metal surface of a car? Why is this process particularly important for vehicles in northern climates, where salt is used on icy roads?
solution:
It is common to dissolve salt on icy roads in northern climates in order to lower the freezing point of water. However, the humidity may cause the car to be corroded, so applying a layer of paint on cars can prevent metal from reacting with the water by isolating them.

