Extra Credit 23
- Page ID
- 83255
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.3.2
For each reaction listed, determine its standard cell potential at 25 °C and whether the reaction is spontaneous at standard conditions.
- \(\mathrm{Mn(s)+Ni^2+(aq) ⟶ Mn^2+(aq)+Ni(s)}\)
- \(\mathrm{3Cu^2+(aq)+2Al(s)⟶2Al^3+(aq)+2Cu(s)}\)
- \(\mathrm{Na(s)+LiNO_3(aq)⟶NaNO_3(aq)+Li(s)}\)
- \(\mathrm{Ca(NO_3)_2(aq)+Ba(s)⟶Ba(NO_3)_2(aq)+Ca(s)}\)
1. Divide redox reaction into their respective half reactions (reduction and oxidation). The reaction being oxidized is the anode while the reaction being reduced is the cathode. Use Ecell = E(cathode) - E(anode) by finding the standard reduction potentials (SRP) of each half reaction. If Ecell > 0, the reaction is spontaneous. If Ecell < 0, the reaction is NOT spontaneous. If Ecell = 0, the reaction is at equilibrium. If you are unable to recall, OIL RIG is a helpful tool to remember which step is oxidation and which is reduction. If the half reaction results in the loss of electrons it is oxidation (Oxidation Is Loose), where as Reduction Is Gain. OIL RIG.
\(\mathrm{Mn(s) → Mn^2+(aq)}\) oxidation (anode) SRP = -1.185 V
\(\mathrm{Ni^2+(aq) → Ni(s)}\) reduction (cathode) SRP = -0.26 V
\(\mathrm{Ecell = Ecathode - Eanode}\)
\(\mathrm{Ecell = -0.26 V - (-1.185 V) = 0.925 V}\)
\(\mathrm{Since Ecell > 0, then ΔG > 0,}\) thus the reaction is spontaneous.
2. \(\mathrm{3Cu^2+(aq) → 2Cu(s)}\) reduction (cathode) SRP = 0.34 V
\(\mathrm{2Al(s) → 2Al^3+(aq)}\) oxidation (anode) SRP = -1.66 V
\(\mathrm{Ecell = Ecathode - Eanode}\)
\(\mathrm{Ecell = 0.34 V - (-1.66 V) = 2 V}\)
\(\mathrm{Ecell > 0,}\) thus reaction is spontaneous.
3. \(\mathrm{Na(s) → NaNO_3(aq)}\) oxidation (anode) SRP = -2.71 V
\(\mathrm{LiNO_3(aq) → Li(s)}\) reduction (cathode) SRP = -3.04 V
\(\mathrm{Ecell = Ecathode - Eanode}\)
\(\mathrm{Ecell = -3.04 V - (-2.71 V) = -0.33}\)
\(\mathrm{Ecell < 0, so ΔG > 0,}\) thus reaction is NOT spontaneous.
4. \(\mathrm{Ca(NO_3)_2(aq) → Ca(s)}\) reduction (cathode) SRP = -2.87 V
\(\mathrm{Ba(s) → Ba(NO_3)_2(aq)}\) oxidation (anode) SRP = -2.912 V
\(\mathrm{Ecell = Ecathode - Eanode}\)
\(\mathrm{Ecell = -2.87 V - (-2.912 V) = 0.042 V}\)
\(\mathrm{Ecell > 0,}\) thus reaction is spontaneous.
Q19.1.21
Predict the products of each of the following reactions and then balance the chemical equations.
- Fe is heated in an atmosphere of steam.
- NaOH is added to a solution of Fe(NO3)3.
- FeSO4 is added to an acidic solution of KMnO4.
- Fe is added to a dilute solution of H2SO4.
- A solution of Fe(NO3)2 and HNO3 is allowed to stand in air.
- FeCO3 is added to a solution of HClO4.
- Fe is heated in air.
- \(\mathrm{3Fe(s)+4H_2O(g)⟶Fe_3O_4(s)+4H_2(g)}\)
- \(\mathrm{3NaOH(aq)+Fe(NO_3)_3(aq) →H_2O Fe(OH)_3(s)+3Na^+(aq)+3NO_3^+(aq)}\)
- \(\mathrm{2CrO_4^2-(aq)+2H_3O^+(aq)⟶2HCrO_4^-(aq) →+2H_2O Cr_2O_7^2-(aq)+3H_2O(l)}\)
- \(\mathrm{6Fe^2+(aq)+Cr_2O_7^2-(aq)+14H_3O^+(aq)⟶6Fe^3+(aq)+2Cr^3+(aq)+2H_2O(l)}\)
- \(\mathrm{Fe(s)+2H_3O^+(aq)+SO_4^2-(aq)⟶Fe^2+(aq)+SO_4^2-(aq)+H_2(g)+2H_2O(l)}\)
- \(\mathrm{4Fe^2+(aq)+O_2(g)+4HNO_3(aq)⟶4Fe^3+(aq)+2H_2O(l)+4NO_3^-(aq)}\)
- \(\mathrm{FeCO_3(s)+2HClO_4(aq)⟶Fe(ClO_4)_2(aq)+H_2O(l)+CO_2(g)}\)
- \(\mathrm{3Fe(s)+2O_2(g)⟶Δ Fe_3O_4(s)}\)
Q19.3.13
[CuCl4]2− is green. [Cu(H2O)6]2+ is blue. Which absorbs higher-energy photons? Which is predicted to have a larger crystal field splitting?
For this problem, it is important to identify the ligand that is attached to the metal (in this case, the metal is Cu) and determine if the ligand is a strong-field ligand or a weak-field ligand. In order to determine if it is a strong-field or weak-field ligand, you must look at the spectrochemical series shown below.

Starting from H2O and going towards the right are all the weak-field ligands. These ligands absorb longer wavelengths than strong-field ligands. Everything from NH3 to the left are all strong-field ligands. They absorb shorter wavelengths than weak-field ligands. Looking at the complex [CuCl4]2-, it can be seen that it has a weak-field ligand. Now looking at the complex [Cu(H2O)6]2+, the H2O ligand is stronger than the Cl ligands in the former metal complex. With this information, you can determine which complex has a larger crystal field splitting. Since we know that [Cu(H2O)6]2+ has a strong-field ligand, it has a low spin complex and low spin complexes have larger crystal field splitting. On the otherhand, [CuCl4]2- has a weak-field ligand which we can connect to a high spin complex that corresponds to a smaller crystal field splitting.
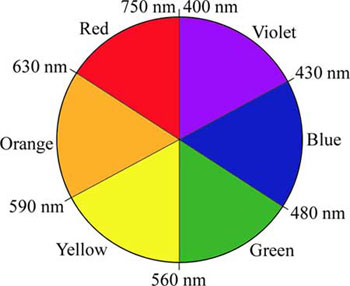
We can find which one absorbs higher energy photons by looking at this color wheel that provides the corresponding wavelengths. Knowing that [CuCl4]2- is seen as the color green, the complex absorbs its complementary color on the color wheel which is red. Looking at [Cu(H2O)6]2+, it is the color blue, which means that the metal complex absorbs orange. The color wheel absorbs the highest energy photons at violet and the lowest energy photons at red. Since [Cu(H2O)6]2+ absorbs orange and [CuCl4]2- absorbs red, it is clear that of the two complexes, [Cu(H2O)6]2+ will absorb the higher energy photons.
Q12.4.13
Both technetium-99 and thallium-201 are used to image heart muscle in patients with suspected heart problems. The half-lives are 6 h and 73 h, respectively. What percent of the radioactivity would remain for each of the isotopes after 2 days (48 h)?
For half-life problems, the equation for the number of half lives is number of half lives (n) = time passed (t) / half life of substance (t1/2).
Using the number of half lives found, you can then input the n number of half lives into the equation (1/2)n [A0] % where [A0] % is the percentage of the initial concentration of the substance. Multiplying out with this equation will result in the percent of the radioactivity that would remain for the isotope.
Technetium-99:
n of half lives of Technetium-99 passed after 2 days = 48/6 = 8
(1/2)8 100% = 0.39% of Technetium-99 left after 48 hours.
Thallium-201:
n of half lives of Thallium-201 passed after 2 days = 48/73 = 0.6575
(1/2)0.6575 100% = 63.39% of Thallium-201 left after 48 hours.
Q21.2.8
The mass of the atom 2311Na is 22.9898 amu.
- Calculate its binding energy per atom in millions of electron volts.
- Calculate its binding energy per nucleon.
1. To calculate binding energy per atom in millions of electron volts, first you must calculate the binding energy of the atom using the equation ΔE = c2Δm, where E is energy, Δm is the mass defect [(total mass of the neutrons and protons) - (mass of the nucleus)], and c is the speed of light (2.998 x 108 m/s).
ΔE = c2Δm
ΔE = [(2.998 x 108 m/s)2] x [11(1.007276 amu) + 12(1.008665 amu)] - (22.9898 amu)]
ΔE = (8.988 x 1016 m/s) x (0.194216 amu)
Convert Δm to kilograms given that 6.022 x 1023 = 1 g and 1000 g = 1 kg.
0.194216 amu x (1 g / 6.022 x 1023 amu) x (1 kg / 1000 g) = 3.225 x 10-28 kg
So now the equation will look like this:
ΔE = (8.988 x 1016 m/s) x (3.225 x 10-28 kg) = 2.898 x 10-11 J
Convert to Millions of electrovolts (MeV)
(2.898 x 10-11 J)/(1.60218 x 10-13) = 180.878 MeV
2. To calculate binding energy per nucleon, first you must calculate the binding energy of the atom using the equation ΔE = c2Δm, where E is energy, Δm is the mass defect [(total mass of the neutrons and protons) - (mass of the nucleus)], and c is the speed of light (2.998 x 108 m/s).
ΔE = c2Δm
ΔE = [(2.998 x 108 m/s)2] x [11(1.007276 amu) + 12(1.008665 amu)] - (22.9898 amu)]
ΔE = (8.988 x 1016 m/s) x (0.194216 amu)
Convert Δm to kilograms given that 6.022 x 1023 = 1 g and 1000 g = 1 kg.
0.194216 amu x (1 g / 6.022 x 1023 amu) x (1 kg / 1000 g) = 3.225 x 10-28 kg
So now the equation will look like this:
ΔE = (8.988 x 1016 m/s) x (3.225 x 10-28 kg) = 2.898 x 10-11 J
Convert to Millions of electrovolts (MeV)
(2.898 x 10-11 J)/(1.60218 x 10-13) = 180.878 MeV
Now that you have found the binding energy, to find the binding energy in joules per nucleon, you must divide 2.898 x 10-11 J by the total number of nucleons.
2.898 x 10-11 J / 23 nucleons = 1.26 x 10-12 J/nucleon
If units need to be in MeV instead of joules compute using MeV / nucleons
180.878 MeV / 23 nucleons = 7.864 MeV/nucleon
Q21.6.3
Iodine that enters the body is stored in the thyroid gland from which it is released to control growth and metabolism. The thyroid can be imaged if iodine-131 is injected into the body. In larger doses, I-133 is also used as a means of treating cancer of the thyroid. I-131 has a half-life of 8.70 days and decays by β− emission.
- Write an equation for the decay.
- How long will it take for 95.0% of a dose of I-131 to decay?
1. Find iodine in the periodic table. Find its atomic number, 53, and we are given the mass number, 133. The iodine will be on one side of the equation while the beta particle will be on the other. The beta particle will have 0 mass but a negative 1 atomic number. Now, you must find an element that when introduced to the side of the equation with the beta emission, will have the mass number and atomic numbers of both sides equal each other. With simple algebra, it is found that the element is Xenon (Xe) with an atomic number of 54 and the mass number of this element must be 133.
13353I → 0-1ß + 13354Xe
2. For the time required for the 95% decay, consider N be the initial amount of sample. Therefore the sample that remains after decay of 95 % is 0.05 N.
The equation for the decay constant (λ) of 18353I is λ = ln 2 / t1/2
We are given the half-life of iodine which is 8.70 days, so λ = ln 2 / 8.70 days. After algebra, λ will equal 0.07967 per day.
To find the time required for the decay, use the equation t = (1/λ)ln(no/nt) the no would be the initial amount of sample before decay and nt would be the amount of sample after decay.
With information that we have found, the equation will be, t = (1/0.07967 per day)ln(N/0.05N) which equals 37.6 days.
So it will take 37.6 days for 95.0% of a dose of I-131 to decay.
Q20.4.9
All reference electrodes must conform to certain requirements. List the requirements and explain their significance.
A reference electrode must ...
- Be good electrical conductor - usually a metal, electrical conductivity determines if the electrode participates in the reaction or if it is inert.
- Have a standard electrode potential of zero - being at zero volts (V) forms the basis one needs to calculate cell potentials using different electrodes or different concentrations.
Q20.8.1
Do you expect a bent nail to corrode more or less rapidly than a straight nail? Why?
A bent nail would corrode more rapidly than a straight nail due to points of stress that allow oxidation to more easily occur on the surface of the nail. Since oxidation occurs more easily, the metal nail will corrode faster than if there were no bends at all.

