Extra Credit 20
- Page ID
- 83251
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)**edits for phase II done in green
Q17.2.9
- An active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
S17.2.9
Answer: Electrode was part of the cathode.
For the problem, remember OIL RIG or Oxidation Is Lose of e- and Reduction Is Gain of e-. In accordance with OIL RIG we know that oxidation occurs at the anode and reduction occurs at the cathode. If not refer to lesson 19.1: Galvanic Cells. In order for something to be gaining mass, mass from electrons, we know that it has to go along with reduction, and reduction occurs at the cathode. This is because electrons get transferred to the cathode through a wire which then get gained by aqueous cations that came from the anode before they were oxidized. These cations then turn into solid form and deposit onto the cathode.
1) Oxidation occurs at anode --> Lose electron(s) -->Metal will be electrolyzed into metal ion(s) and electron(s)
--> Reduce mass
2) Reduction occurs at cathode --> Gain electron(s) --> Metal ion(s) will combine with electron(s) --> "Form metal(s)"
--> Gain mass
Q19.1.18
Describe the electrolytic process for refining copper.
Q19.1.18
The process for refining copper is a very common chemical way in order to get very pure copper from the process of electrolysis. First we need to know what electrolysis is. Electrolysis is a way to chemically decompose certain ores by passing an electrical current through a liquid or solution that already contains the ions of the wanted material.
Since copper is always found in an ore, it is almost always going to be combined with other impurities such as gold or iron. The most common ore for copper is known as a Chalcopyrite but this is a much more complicated process. We will focus on an easier, very similar way to refine copper.
Electrolytic refining
The purification uses an electrolyte of copper(II) sulfate solution, impure copper anodes, and strips of high purity copper for the cathodes.
The diagram shows a simplified version that is very similar
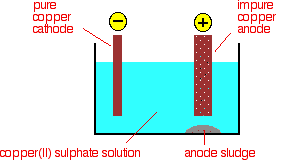
At the cathode, copper(II) ions are deposited as copper.
![]() \[ Cu^{2+}(aq) + 2e^- \rightarrow Cu(s) \tag{5a}\]
\[ Cu^{2+}(aq) + 2e^- \rightarrow Cu(s) \tag{5a}\]
At the anode, copper goes into solution as copper(II) ions.
![]() \[ Cu (s) \rightarrow Cu^{2+} (aq) + 2e^- \tag{6a}\]
\[ Cu (s) \rightarrow Cu^{2+} (aq) + 2e^- \tag{6a}\]
For every copper ion that is deposited at the cathode, in principle another one goes into solution at the anode. The copper(II) cation in the solution comes from the the copper breaking its bond with sulfate. That leaves sulfate anions in the solution along with the copper cations that eventually get reduced at the cathode. The concentration of the solution should stay the same.
All that happens is that there is a transfer of copper from the anode to the cathode. The cathode gets bigger as more and more pure copper is deposited; the anode gradually disappears.
Q19.3.10
Would you expect the complex [Co(en)3]Cl3 to have any unpaired electrons? Any isomers?
Q19.3.10
\(\ce{[Co(en)3]Cl3}\) does not have any unpaired electrons.
In order to figure this out you need to calculate the oxidation state of Cobalt. Since there are three chlorine ions with a negative charge, and the overall charge of the system needs to be neutral or zero, Cobalt needs to have a positive three charge. Then go over to the periodic table and determine the number of d electrons Cobalt three positive has. Count three transition metals back to Chromium and then add the two 4s orbitals, back to Fr or Iron. Since iron is the 6th transition metal in the 3d row, there are 6d electrons.
Next, look at the spectrochemical series and take note that (en) is a pretty strong ligand. This mean that since it is a strong field ligand it will have a low spin since the pairing energy is less than the splitting energy. Now all you have to do is fill in the diagram and since this is an octrahderal complex with six ligands it will have two spaces on the top and three on the bottom. All three spaces on the bottom with be full since there are 6 electron filling the bottom row. This means that there are no unpaired electrons for \(\ce{[Co(en)3]Cl3}\). The fact that there are no unpaired electrons also reveals that the ion is diamagnetic.
The complex does not have any geometric isomers because all of the ligands are the same things but since the mirror image is nonsuperimposable, it has an optical isomer. Another way to tell that it has optical isomers: the complex is optically active because it has no line of symmetry.
Q12.4.10
The reaction of compound A to give compounds C and D was found to be second-order in A. The rate constant for the reaction was determined to be 2.42 L/mol/s. If the initial concentration is 0.500 mol/L, what is the value of t1/2?
S12.4.10
Since you are told that this is a second order reaction, are given the rate constant, or k value, and the initial concentration, you can plug the values straight into this equation:
This is the equation to determine the half life of a second order compound. k= 2.42 L/mol/s and [Ao] = .5 M... units cancel out to give seconds as the final unit.
\[t_{1/2}=\dfrac{1}{(k)[A]_0}\]
\[t_{1/2}=\dfrac{1}{(2.42)(0.500)}\]
\[t_{1/2}= 0.826 \,s\]
Q21.2.5
Write the nuclide notation, including charge if applicable, for atoms with the following characteristics:
- 25 protons, 20 neutrons, 24 electrons
- 45 protons, 24 neutrons, 43 electrons
- 53 protons, 89 neutrons, 54 electrons
- 97 protons, 146 neutrons, 97 electrons
S21.2.5
Nuclide notation:
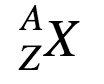
A is the atomic mass, Z is the atomic number (number of protons), and X is the symbol.
1. \(\ce{^{55}_{25}Mn^+}\) *Phase I answer: A = 55, A(atomic mass) should be 45 because 25 protons + 20 neutrons = 45
2. \(\ce{^{69}_{45}Rh^2+}\)
3. \(\ce{^{142}_{53}I^-}\)
4. \(\ce{^{243}_{97}Bk}\)
For each answer you have to determine the four different components. The name of the compound is dictated by how many protons are in the element. For example, in number one there are 25 protons, therefore this element is Manganese based off of the periodic table. The number of protons also determines the bottom left number, which is known as the atomic number. The top left number, also known as the mass number, is the addition of both the number of neutrons and protons. For #1 this would make the mass number 25 + 20 or 55. Next you need to determine the charge of the element, and this is done so by determining whether the element is positively or negatively charged, or neutral. If there are more electrons than protons it will have a negative charge. If there are more protons than electrons it will be positively charge, and if both are equal it is neutral. For #1 the element is positively charge because there is one more proton than neutron.
Q21.5.8
The mass of a hydrogen atom (11H)(11H) is 1.007825 amu; that of a tritium atom (31H)(13H) is 3.01605 amu; and that of an α particle is 4.00150 amu. How much energy in kilojoules per mole of He24He24produced is released by the following fusion reaction: H11+H13⟶He24H11+H13⟶He24.
Q21.5.8
First find the mass defect from the given equation.
You find the mass defect by sutracting the products mass for the reactants mass as shown below.
* (1.007825+3.01605) - 4.00150 = .022375 amu, not .04475 amu as was calculated in phase I
\(\begin{align}
\mathrm{mass\: defect} & \mathrm{= (8.003\,amu) - (2.01565\,amu + 6.0321\,amu)}\\
& \mathrm{= -0.04475\,amu}
\end{align}\)
From the mass defect you can then plug it into the mass energy equivalence equation to find the KJ/mol
\(\mathrm{E=mc^2}\)
\(\mathrm{m= -0.04475\, g \times \dfrac{1\,kg}{1000\,g} = -4.475 \times 10^{-5}}\)
\(\mathrm{E= (-4.475 \times 10^{-5})(3.00 \times 10^8)^2 = 4.0275 \times 10^{12}\, kg\, m^2/s^2}\)
*E = (3.71545584e-29 kg)(3.00e8 m/s)^2 = 3.34e17 Joules = 3.34e14 kj
times by avagodros number to get the amount of energy per mol
* avogadro's number is 6.022e23, not 6.022e-23 as used below
\(\mathrm{ 4.0275\times 10^{12})(6.022 \times 10^-23) = 2.425 \times 10^{12}\, KJ\mol}\)
* 3.34e14 kj(6.022e23) = 2.01e38 kj/mol
Q20.4.6
Explain why E° values are independent of the stoichiometric coefficients in the corresponding half-reaction.
Q20.4.6
Because electrical potential is the energy needed to move a charged particle in an electric field, standard electrode potentials for half-reactions are intensive properties and do not depend on the amount of substance involved. Consequently, E° values are independent of the stoichiometric coefficients for the half-reaction, and, most important, the coefficients used to produce a balanced overall reaction do not affect the value of the cell potential Because rather, they are dependent on temperature, concentration, and pressure. Refer to 19.2: Standard Electrode Potentials for a more in depth example.
Q20.7.4
Why are galvanic cells used as batteries and fuel cells? What is the difference between a battery and a fuel cell? What is the advantage to using highly concentrated or solid reactants in a battery?
Q20.7.4
Galvanic cells are used a batteries and fuel cells because the are both self-contained and portable. This means that they are easy to take around because they are comprised of more than one voltaic cell creating a galvanic cell which is the combination of voltaic which is also known as a battery. Galvanic cells are also used as batteries and fuel cells because the redox reactions involve provide the electrons that create a voltage when transferred that powers electricity. The difference between a battery and a fuel cell is that a battery is spontaneous, meaning that it contains all of the necessary products to produce the needed electricity. A fuel cell is a galvanic cell that needs some sort of external supply to generate the electricity. The advantage to using a highly concentrated or solid reactants in a battery allows for more electrons to be transferred and create more electricity for a longer period of time. For more information refer to 19.1: Galvanic Cells
PHASE II (by Marialisa Grassa) :

a) The process of coating a metal with another metal through electrolysis is called electroplating. The electrode that gains mass during this process is the cathode. That is because in order for the metal from the other electrode to lose the mass of the metal, it must pull electrons from it. The new positively charged metal ions flow through solution toward the cathode where they attract the previously pulled electrons and convert back into solid form while depositing itself on the electrode. Because the ions gained electrons, they were reduced, therefore making the electrode that gains mass the cathode.
b) The electrode that loses mass is the anode because the metal that it is composed of loses electrons and are converted into cations that leave the electrode and are released into the solution as cations that eventually deposit onto the cathode. Since the molecules lost electrons, they were oxidized at the electrode, making it the anode.

During electrolysis, copper can get refined from its ore. An impure copper anode is put in an electrolytic copper solution such as copper(II) sulfate along with a copper cathode. After a current is passed through the system, the solution splits into copper cations and sulfate anions. The copper cations are oxidized and released at the anode as well. The positively charged copper passes through the aqueous solution and makes its way to the cathode where it is reduced to form pure, metal copper and deposits on the cathode.

a) To determine if the complex has any unpaired electrons, we need to know some information about the transition metal, Cobalt and its ligand, (en). By looking at the spectrochemical series, we can observe that en is a neutral, strong field ligand. This means the complex is low spin. It should also be known that en is a bidentate ligand, meaning it makes 2 bonds per en molecule with its transition metal. We can conclude that since there are three en ligands, the complex has a coordination number of 6, giving it an octahedral geometry. Now we can make an octahedral orbital energy diagram:
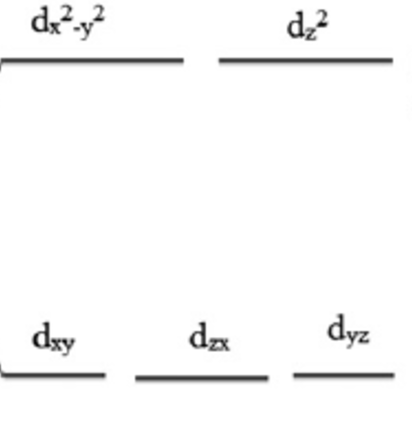
Since en is a neutral ligand and chlorine has an oxidation number of -1, Cobalt in this complex ion has a +3 oxidation number. We look at the periodic table to determine that Cobalt's electronic configuration is [Ar] 3d6. The 6 electrons occupy the lower t2g d orbitals because the complex is low spin.
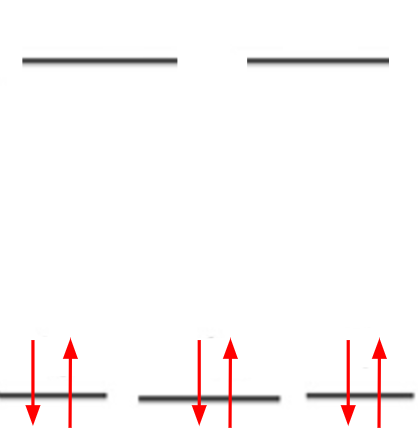
From this we can see that the complex has no unpaired electrons.
b) The complex has no geometric isomers because all of its ligands are of the same type of molecule; however, it has two optical isomers because the mirror images of the complex are non superimposable, making them chiral enantiomers. You can also tell that it is an optical isomer because the molecule has no line of symmetry because of the way that en binds to cobalt as a bidentate ligand. Complex ions with no line of symmetry have optical isomers.

Because it is given that the reaction is second order, we use the corresponding equation to determine half life:

We then plug in the given values for the rate constant (k = 2.42 L/mol/s) and the initial concentration ([Ao] = .5 M).

The equation yields a half life of .826 seconds.

Nuclide notation is written in the format:
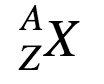
A= Atomic mass (number of protons + neutrons)
Z= Atomic number (number of protons)
X= Atomic symbol
* charge is written as a superscript after the atomic symbol
a. A= 25 protons + 20 neutrons = 45, Z = 25 (corresponding symbol is Mn), charge = 25 protons- 24 electrons = +1

b. A= 45 protons + 24 neutrons = 69, Z= 45 (corresponding symbol is Rh), charge = 45 protons - 43 electrons = +2
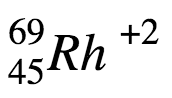
c. A= 53 protons + 89 neutrons = 142, Z= 53 (corresponding symbol is I), charge = 53 protons - 54 electrons = -1

d. A= 97 protons + 146 neutrons = 243, Z= 97(corresponding symbol is Bk), charge = 97 protons - 97 electrons = 0


***Wrong answer in Phase I
To calculate binding energy, we must use the equation E= mc^2 (c= speed of light = 3.00e8 m/s)
The change in mass that we plug in for m is the sum of the mass of the reactants minus the mass of the helium atom:
(1.007825 + 3.01605) - 4.00150 = .022375 amu
converting this to kg gives us 3.71545584e-29 kg
After plugging in this value for m, we get:
E = (3.71545584e-29 kg)(3.00e8 m/s)^2 = 3.34e17 Joules
3.34e17 j converts to 3.34e14 kj
Now multiply by avogadros number to get wanted units:
3.34e14 kj* 6.022e23 = 2.01e38 kj/mol

Standard reduction potentials are independent of stoichiometric coefficients in a corresponding half reaction because the value represents the relative ability for a compound to get reduced. This is dependent on only temperature, pressure, and concentration at standard conditions (298k, 1atp, and 1 M, respectively) rather than stoichiometric coefficients.

Galvanic cells are used as batteries and fuel cells because redox reactions allow an oxidized substance to donate electrons that power electricity spontaneously.
A fuel cell relies on an external source for energy while a battery is self contained.
A highly concentrated, solid reactant is better to use for a battery because it means there are more atoms to oxidize and receive electrons from to power electricity.

