Extra Credit 10
- Page ID
- 83240
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.9
a) Why is it not possible for hydroxide ion (OH−) to appear in either of the half-reactions or the overall equation when balancing oxidation-reduction reactions in acidic solution?
b) Why is it not possible for hydrogen ion (H+) to appear in either of the half-reactions or the overall equation when balancing oxidation-reduction reactions in basic solution?
S17.1.9
a) It would not be possible because the hydroxide ion (OH-) would pair up with an equal amount of H+ ions present in the reaction. They would then form additional water molecules and be canceled with the water molecules already present in the opposite side of the reaction. When one adds the two reactions are together,. common terms on either side of the reaction arrow are eliminated. Therefore, no OH- ions should be present in the overall balanced redox equation. Since it may be in an acidic solution, there will be an excess amount of H+ ions present in the final solution/equation with no hydroxide ions present.
b) In basic solution, [OH−] > 1 × 10−7 M > [H+]. Hydrogen ion cannot appear as a reactant because its concentration is essentially zero. If it were produced, it would instantly react with the excess hydroxide ion to produce water. Thus, hydrogen ion should not appear as a reactant or product in basic solution. Since it is a basic solution, there will still be an excess amount of hydroxide ions present in the final solution/equation. If one were to balance reactions in basic solution, after balancing the atoms and oxidation numbers, first treat the reactions as an acidic solution and then add OH- ions to balance the H+ ions in the half reactions (which would give H2O).
Q19.1.8
The following reactions all occur in a blast furnace. Which of these are redox reactions?
a) \(3Fe_2O_3(s)+CO(g)⟶2Fe_3O_4(s)+CO_2(g)\)
b) \(Fe_3O_4(s)+CO(g)⟶3FeO(s)+CO_2(g)\)
c) \(FeO(s)+CO(g)⟶Fe(l)+CO_2(g)\)
d) \(C(s)+O_2(g)⟶CO_2(g)\)
e) \(C(s)+CO_2(g)⟶2CO(g)\)
f) \(CaCO_3(s)⟶CaO(s)+CO_2(g)\)
g) \(CaO(s)+SiO_2(s)⟶CaSiO_3(l)\)
S19.1.8
a) This is a redox reaction because Fe undergoes reduction (gains electrons), +3 to +8/3, while C undergoes oxidation (loses electrons), -1 to +4.
b) This is a redox reaction because Fe undergoes reduction (gains electrons), +8/3 to +2, while C undergoes oxidation (loses electrons), -1 to +4.
c) This is a redox reaction because Fe undergoes reduction (gains electrons), +2 to 0, while C undergoes oxidation (loses electrons), -1 to +4.
d) This is a redox reaction because C undergoes oxidation (loses electrons), 0 to +4, while O undergoes reduction (gains electrons), 0 to -2.
e) This is a redox reaction because C undergoes reduction (gains electrons), 0 to -1, while O undergoes oxidation (loses electrons), -2 to +1.
f) This is not a redox reaction because Ca remains with a +2 charge, O remains with a -2 charge, and C remains with a +4 charge.
g) This is not a redox reaction because Ca remains with a +2 charge, Si remains with a +4 charge, and O remains with a -2 charge.
For reduction, the number gets smaller. For oxidation, the number gets bigger.
Q19.2.10
Draw the geometric, linkage, and ionization isomers for [CoCl5CN][CN].
S19.2.10
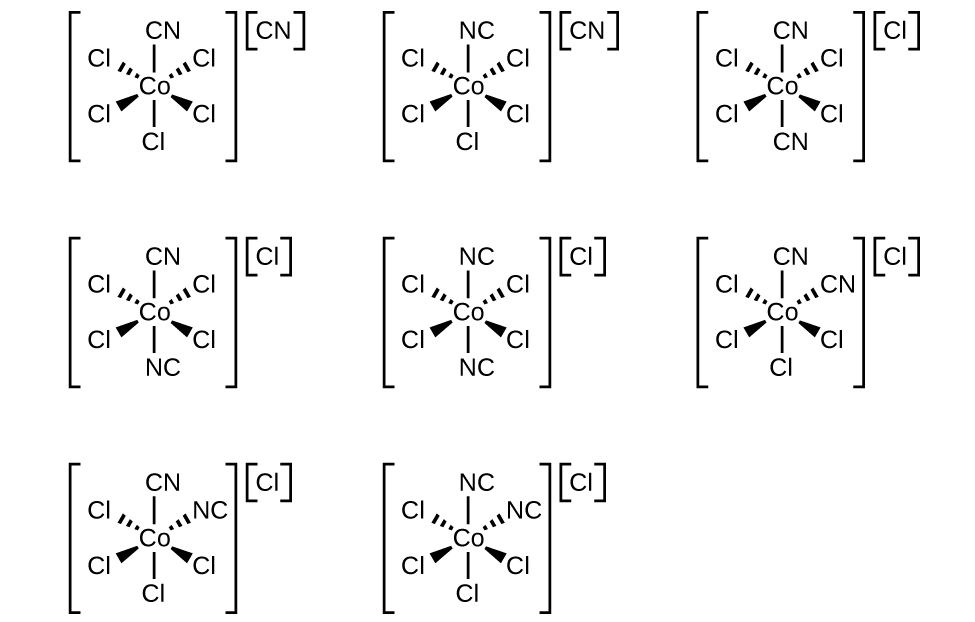
Recall first what geometric, linkage, and ionization isomers are as that is knowledge you must have to answer this question.
Geometric isomers are metal complexes that differ only in which ligands are adjacent to one another (cis) or directly across from one another (trans) in the coordination sphere. No matter which view point you have on the molecule, the molecule does not change.
Linkage isomers are two or more coordination compounds in which the donor atom of at least one of the ligands is different. This can exist when the compound contains that can bond to a metal atom in two or more different ways.
Ionization isomers have the same components but different geometries. This occurs when the ions inside and outside of the coordination sphere interhcange.
Now to solve the problem:
Technically, the coordination sphere (first set of brackets) will have no geometric isomers since it follows the octahedral model ML5X. (M=Co, L5=Cl5, X=CN).
But since it is paired up with a CN- ligand (counter ion), more geometric isomers are possible. As a result, the coordination compound in structures 3-9 pictured above (the 3rd one on the first line, and the rest on the second and third lines) follow the octahedral form, ML4X2 (M=Co, L4=Cl4, X2=(CN)2) and has both cis- and trans- geometric isomers. After one Cl- ligand switches places with a CN- ligand, the coordination complex (surrounded by brackets) has both geometric isomers, cis- and trans-, since the last three structures (3rd one on the second line, and the two on the last line) have two CN- ligands that are located 90o apart from each other and are on the same side (cis-) while two Cl- ligands are located 180o across from each other (trans-). The 3rd structure on the first line and the first 2 structures on the second line have both CN- ligands lying 180o across from each other which forms a trans- isomer while the rest of the Cl- ligands lie 90o from each other on the same side which forms two cis- isomers.
This molecule also has linkage isomers in which the Nitrogen from the CN- ligand forms a bond with the central atom, Cobalt. Likewise, the Carbon atom from the CN- ligand forms a bond with the central atom, Cobalt.
This structure can form an ionization isomer in which one CN- ligand is directly bound to the central metal atom while one of the Cl- ligands is attached to the entire coordination complex. Likewise, a Cl- ligand can also be directly bound to the central metal atom while one CN- ligand is attached to the entire coordination complex.
Q12.3.23
In the reaction \[2NO+Cl_2→2NOCl\]
the reactants and products are gases at the temperature of the reaction. The following rate data were measured for three experiments:
| Initial p{NO} | Initial p{Cl2} | Initial rate |
|---|---|---|
| (atm) | (atm) | (moles of A consumed atm sec-1) |
| 0.50 | 0.50 | 5.1 x 10-3 |
| 1.0 | 1.0 | 4.0 x 10-2 |
| 0.50 | 1.0 | 1.0 x 10-2 |
a) From these data, write the rate equation for this gas reaction. What order is the reaction in NO, Cl2, and overall? * ([ ] (brackets) denote { } (braces))
b) Calculate the specific rate constant for this reaction.
S12.3.23
a) Use the values from the second and third experiments for the initial values of p{NO} and the corresponding initial rates.
\[p[{NO}]={1.0}^x/{0.50}^x=(4.0 \,x\, 10^{-2}/1.0 \,x\, 10{-2})\]
Simplify.
\[p[{NO}]=2x=4\]
Divide by 2.
\[x=2; [{NO}]^2\]
Plug in values from the first and third experiments for the initial values of p{Cl2} and the corresponding initial rates.
\[p[{Cl_2}]=[{0.50}]^x/[{1.0}]^x=(5.1 \,x\, 10^{-3}/1.0 \,x\, 10^{-2})\]
Simplify.
\[p[{Cl_2}]=0.50^x=0.51\]
\[x=1; [{Cl_2}]^1\]
Overall order of the reaction: 2 + 1 = 3
b) Plug in values from the first, second, or third experiment only to figure out the value of k. In this problem, we use the values from experiment 1 for both p{NO} and p{Cl2}.
\[p[{NO}]=p[{0.50}]; p[{NO}]^2=p[{0.50}]^2=0.25 atm\]
\[p[{Cl_2}]=p[{0.50}]; p[{Cl_2}]^1=p[{0.50}]^1=0.50 atm\]
\[k(p[{NO}])(p[{Cl_2}])=5.1 x 10^{-3} mol \,x\, atm/sec\]
\[k(0.25 atm)(0.50 atm)=5.1 x 10^{-3} mol \,x\, atm/sec\]
\[k(0.125 atm^2)=5.1 x 10^{-3} mol /atm \,x\, sec\]
k=0.0408 mol/atm x sec
Q12.7.3
Consider this scenario and answer the following questions: Chlorine atoms resulting from decomposition of chlorofluoromethanes, such as CCl2F2, catalyze the decomposition of ozone in the atmosphere. One simplified mechanism for the decomposition is:
\[O_3 \xrightarrow{sunlight} O_2 + O\\ O_3 + Cl ⟶ O_2 + ClO\\ ClO + O ⟶ Cl + O_2\]
a) Explain why chlorine atoms are catalysts in the gas-phase transformation:
\[2O_3⟶3O_2\]
b) Nitric oxide is also involved in the decomposition of ozone by the mechanism: \[O_3 \xrightarrow{sunlight} O_2 + O\\ O_3 + NO ⟶ NO_2 + O_2\\ NO_2 + O ⟶ NO + O_2\]
Is NO a catalyst for the decomposition? Explain your answer.
S12.7.3
a) Chlorine atoms are a catalyst because they react in the second step but are regenerated in the third step. Thus, they are not used up, which is a characteristic of catalysts.
b) NO is a catalyst for the decomposition reaction because Chlorine atoms are a catalyst since they react in the second step but are regenerated in the third step. Thus, they are not used up, which is a characteristic of catalysts.
Q21.4.26
Isotopes such as 26Al (half-life: 7.2 × 105 years) are believed to have been present in our solar system as it formed, but have since decayed and are now called extinct nuclides.
a) 26Al decays by β+ emission or electron capture. Write the equations for these two nuclear transformations.
b) The earth was formed about 4.7 × 109 (4.7 billion) years ago. How old was the earth when 99.999999% of the 26Al originally present had decayed?
S21.4.26
a) \[β^+ emission: {^{26}_{13}Al\rightarrow^{26}_{12}Mg^*+^0_{+1}\beta^++neutrino}\]
(* is meant to signify that the nucleus is unstable and will also decay)
\[Electron\, capture: {^{26}_{13}Al\rightarrow^0_{-1}e+^{26}_{14}Si}\]
b) Use the equations: \[N=\lambda t\,and\, In(\frac{N_t}{N_0})=-\lambda t\]
Find the givens: \[t_{1/2}=7.2\,x\,10^5\,years , N_0=100%, N_t=100%-99.999999%\]
\[t_{1/2}=\frac{In2}{\lambda }; {\lambda }=\frac{In2}{7.2\,x\,10^5}\]
Plug the givens into the equation.
\[In(\frac{100-99.999999}{100})-\frac{In2}{7.2\,x\,10^5}=t\]
Simplify to find t.
\[t=-18.4207\,years\]
Subtract the amount of years Earth was formed (4.7 billion years) from calculated t (time).
\[-18.4207-4.7\,x\,10^9=-4.7\,x\,10^9\,billion\,years\]
Q20.3.13
For each galvanic cell represented by these cell diagrams, determine the spontaneous half-reactions and the overall reaction. Indicate which reaction occurs at the anode and which occurs at the cathode.
a) \(Zn(s)∣Zn^{2+}(aq) ∥ H^+(aq)∣H_2(g), Pt(s)\)
b) \(Ag(s)∣AgCl(s)∣Cl^−(aq) ∥ H^+(aq)∣H_2(g)∣Pt(s)\)
c) \(Pt(s)∣H_2(g)∣H^+(aq) ∥ Fe^{2+}(aq), Fe^{3+}(aq)∣Pt(s)\)
S20.3.13
a) \[reduction\, half-reaction: 2H+(aq) + 2e− → H_2(aq); cathode\]
\[oxidation\, half-reaction: Zn(s) → Zn2+(aq) + 2e−; anode\]
(Cancel out the 2e- from the reactant side of the reduction reaction and the 2e- from the product side of the oxidation reaction)
\[overall: Zn(s) + 2H^+(aq) → Zn^{2+}(aq) + H_2(aq)\]
b) \[reduction\, half-reaction: AgCl(s) + e^− → Ag(s) + Cl^−(aq); cathode\]
(Multiply reduction half-reaction by 2): \[2AgCl(s) + 2e^- → 2Ag(s) + 2Cl^-(aq)\]
\[oxidation\, half-reaction: H_2(g) → 2H^+(aq) + 2e^−; anode\]
(Cancel out the 2e- from the reactant side of the reduction reaction and the 2e- from the product side of the oxidation reaction)
\[overall: 2AgCl(s) + H_2(g) → 2H^+(aq) + 2Ag(s) + 2Cl^−(aq)\]
c) \[reduction\, half-reaction: Fe^{3+}(aq) + e^− → Fe^{2+}(aq); cathode\]
(Multiply reduction half-reaction by 2): \[2Fe^{3+}(aq) + 2e^- → 2Fe^{2+}(aq)\]
\[oxidation\, half-reaction: H_2(g) → 2H^+(aq) + 2e^−; anode\]
(Cancel out the 2e- from the reactant side of the reduction reaction and the 2e- from the product side of the oxidation reaction)
\[overall: 2Fe^{3+}(aq) + H_2(g) → 2H^+(aq) + 2Fe^{2+}(aq)\]
Q20.5.25
Given the following biologically relevant half-reactions, will FAD (flavin adenine dinucleotide), a molecule used to transfer electrons whose reduced form is FADH2, be an effective oxidant for the conversion of acetaldehyde to acetate at pH 4.00?
\[acetate + 2H^+ +2e^− → acetaldehyde + H_2O\qquad\,E° = −0.58 V\]
\[FAD + 2H^+ +2e^− → FADH_2\qquad\,E° = −0.18 V\]
S20.5.25
FAD will be an effective oxidant for the conversion of acetaldehyde to acetate at a pH of 4.00 because it has a higher reduction potential than the reduction potential of acetate. High, positive E° values are good oxidizing agents.
(oxidant or oxidizing agent = gets reduced)
\[FAD \, (-0.18V) > acetate \, (-0.58V)\]

