Extra Credit 24
- Page ID
- 83419
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.8A
Find \(\mathrm{\Delta G^\circ}\) by combining the following half reactions to calculate the \(\mathrm{E^\circ}\) cell at:
\(\begin{align}
& \mathrm{Ag^{2+} + 2e^- \rightarrow Ag(s)} \\
& \mathrm{Ag^{2+}(aq) + e^- \rightarrow Ag^+(aq)} \quad & & \mathrm{E^\circ= +1.98\,V} \\
& \mathrm{Ag^+(aq) + e^- \rightarrow Ag(s)} \quad & & \mathrm{E^\circ= +0.800\,V}\end{align}\)
S19.8A
\(\begin{align}
& \mathrm{Ag^{2+}(aq) + e^- \rightarrow Ag^+(aq)} \quad & & \mathrm{\Delta G^\circ= -(1 \times F \times (1.98\,V))} \\
& \underline{\mathrm{Ag^+ (aq) + e^- \rightarrow Ag (s)}}\quad & & \underline{\mathrm{\Delta G^\circ= -(1 \times F \times (0.800\,V))}} \\
& \mathrm{Ag^{2+} (aq) + 2e^- \rightarrow Ag(s)} \quad & & \mathrm{\Delta G^\circ= -(1.98\,V)F - (0.800\,V)F} \\
& \, \quad & & \mathrm{\Delta G^\circ= (-2.78\,V)F}\end{align}\)
\(\mathrm{\Delta G^\circ= -nFE^\circ_{cell}}\)
\(\mathrm{(-2.78\,V)F = -2FE^\circ_{cell}}\)
\(\mathrm{E^\circ_{cell}=\dfrac{(-2.78\,V)F}{-2F}}\)
\(\mathrm{E^\circ_{cell}= +1.39\,V}\)
Explanation: In order to find the \(\mathrm{\Delta G^\circ}\), it is important to use the equation \(\mathrm{\Delta G^\circ= -nFE^\circ_{cell}}\). This will be given, but it is important to know what this equation does. The "n" in the equation represents the number of electrons that are being transferred throughout the reaction. The "F" is Faraday's constant which will be provided on an answer sheet. The cell potential will be given on a standard reduction potential chart. The "n" for these equations will be 1 because there is one electron that is being transferred throughout the half-reactions. The half-reactions can be added together in order to give the total \(\mathrm{\Delta G^\circ}\). This can be plugged into the equation that was previously mentioned with the "n" value of 2 because 2 electrons are being moved throughout the entirety of the equation. This will result in a cell potential of +1.39 V.
Q19.65A
Assuming all reactants and products are currently in their standard states, determine which of the following reactions occur spontaneously and which can occur only through the use of electrolysis. Also, for those that require electrolysis, determine what the minimum voltage required is.
- \(\mathrm{Cu(s) + Zn^{2+}(aq) \rightarrow Cu^{2+}(aq) + Zn(s)}\)
- \(\mathrm{2Al + 3Cu^{2+} \rightarrow 3Cu + 2Al^{3+}}\)
- \(\mathrm{Zn + Cl_2(g) \rightarrow ZnCl_2(aq)}\)
- \(\mathrm{2Fe^{3+} + 2Cl^- \rightarrow 2Fe^{2+} + Cl_2(g)}\)
S19.65A
- Ox: \(\mathrm{Cu \rightarrow Cu^{2+} + 2e^- \hspace{47 pt}\quad E^\circ= -0.340\:V}\)
Red: \(\mathrm{Zn^{2+} + 2e^- \rightarrow Zn \hspace{42 pt}\quad E^\circ= -0.763\:V}\)
------------------------------------------------------------------------------
Overall: \(\mathrm{Cu + Zn^{2+} \rightarrow Cu^{2+} + Zn \quad E^\circ=-1.103}\)
Since voltage is negative, it requires electrolysis with an applied voltage of greater than 1.103 V
Explanation: The overall reaction should first be split into the oxidation and reduction half-reactions. The different reactions can be determined by the electrons being transferred. Since the balanced half-reaction with "Zn" contains electrons on the left-hand side of the reaction, this indicates that the reaction is going to be reduction since it is gaining electrons. The balanced half-reaction with "Cu" contains electrons on the right-hand side of the reaction, indicating that the reaction is going to be oxidation since it is losing electrons. The cell potential values are given from the standard reduction potential chart. To obtain the overall value of the cell potential, you simply add the standard reduction potentials together. The voltage of this problem is going to be negative, indicating that the reaction is going to be non-spontaneous. Electrolysis is going to be needed in order for the reaction to run. The amount of electricity that is going to be needed is going to be more than the overall cell potential.
Red: \(\mathrm{Cu^{2+} + 2e^- \rightarrow Cu \hspace{38.5 pt} \quad E^\circ=0.340\:V}\)
Ox: \(\mathrm{Al \rightarrow Al^{3+} + 3e^- \hspace {49 pt}\quad E^\circ= 1.676\:V}\)
------------------------------------------------------------------------------
Overall: \(\mathrm{Cu^{2+} + Al \rightarrow Cu + Al^{3+} \quad E^\circ= 2.016\:V}\)
This is a spontaneous reaction under standard conditions because it has positive V.
Explanation: The overall reaction should first be split into the oxidation and reduction half-reactions. The different reactions can be determined by the electrons being transferred. Since the balanced half-reaction with "Cu" contains electrons on the left-hand side of the reaction, this indicates that the reaction is going to be reduction since it is gaining electrons. The balanced half-reaction with "Al" contains electrons on the right-hand side of the reaction, indicating that the reaction is going to be oxidation since it is losing electrons. The cell potential values are given from the standard reduction potential chart. To obtain the overall value of the cell potential, you simply add the standard reduction potentials together. The voltage of this problem is going to be positive, indicating that the reaction is going to be spontaneous. Electrolysis is not going to be needed in order for the reaction to run.
- Red: \(\mathrm{Cl_2 + 2e^- \rightarrow 2Cl^- \hspace{41 pt} \quad E^\circ= 1.358\:V}\)
Ox: \(\mathrm{Zn \rightarrow Zn^{2+} + 2e^- \hspace{49 pt} \quad E^\circ= 0.763\:V}\)
------------------------------------------------------------------------------
Overall: \(\mathrm{Cl_2 + Zn \rightarrow 2Cl^- + Zn^{2+} \quad E^\circ= 2.121\:V}\)
This is a spontaneous reaction under standard conditions because it has positive V.
Explanation: The overall reaction should first be split into the oxidation and reduction half-reactions. The different reactions can be determined by the electrons being transferred. Since the balanced half-reaction with "Cl" contains electrons on the left-hand side of the reaction, this indicates that the reaction is going to be reduction since it is gaining electrons. The balanced half-reaction with "Zn" contains electrons on the right-hand side of the reaction, indicating that the reaction is going to be oxidation since it is losing electrons. The cell potential values are given from the standard reduction potential chart. To obtain the overall value of the cell potential, you simply add the standard reduction potentials together. The voltage of this problem is going to be positive, indicating that the reaction is going to be spontaneous. Electrolysis is not going to be needed in order for the reaction to run.
- Red: \(\mathrm{Fe^{3+} + e^- \rightarrow Fe^{2+} \hspace{62 pt}\quad E^\circ= 0.771\:V}\)
Ox: \(\mathrm{2Cl^- \rightarrow Cl_2(g) + 2e^- \hspace{55 pt}\quad E^\circ= -1.358\:V}\)
-------------------------------------------------------------------------------------
Overall: \(\mathrm{Fe^{3+} + 2Cl^- \rightarrow Fe^{2+} + Cl_2(g) \quad E^\circ= -0.587\:V}\)
Since the V is negative, it requires electrolysis with an applied V of greater than 0.587 V.
Explanation: The overall reaction should first be split into the oxidation and reduction half-reactions. The different reactions can be determined by the electrons being transferred. Since the balanced half-reaction with "Fe" contains electrons on the left-hand side of the reaction, this indicates that the reaction is going to be reduction since it is gaining electrons. The balanced half-reaction with "Cl" contains electrons on the right-hand side of the reaction, indicating that the reaction is going to be oxidation since it is losing electrons. The cell potential values are given from the standard reduction potential chart. To obtain the overall value of the cell potential, you simply add the standard reduction potentials together. The voltage of this problem is going to be negative, indicating that the reaction is going to be non-spontaneous. Electrolysis is going to be needed in order for the reaction to run. The amount of electricity that is going to be needed is going to be more than the overall cell potential.
Q21.5A
- The complex ion \(\ce{[Mn(CN)4]^2-}\) is paramagnetic with 1 unpaired electron. Use the crystal field theory to explain its possible structure.
- Would you expect \(\ce{[Mn(NH3)4]^2+}\) to be diamagnetic or paramagnetic? Can you use this information about magnetic properties to help you determine whether the structure is tetrahedral or square planar?
S21.5A
- The electron configuration of \(\ce{Mn^2+}\) is [Ar]3d5. Because the complex ion is paramagnetic, there will be unpaired electrons. This compound has a coordination number of 4 so it could either be square planar or tetrahedral.
Explanation: When determining the electron configuration of transition metals, it is important to look at the charge. Since the charge is "+2", that indicates that it is going to lose 2 electrons. This affects the electron configuration by first removing the "s-orbital" electrons. These electrons are always the first ones to be removed from transition metal complex ions. The term "paramagnetic" indicates that there will be unpaired electrons. You can tell that the coordination number will be 4 because there are 4 "CN" molecules attached to the transition metal. The crystal field theory indicates that if the coordination number is 4, then it is possible for the structure to be either tetrahedral or square planar.
b. \(\ce{Mn^2+}\): [Ar]d5; 5 electrons=paramagnetic
\(\ce{NH3}\) is a strong field; it has a large crystal field splitting, which means it has a low spin according to the spectrochemical series.
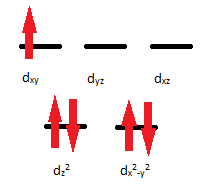
For review on this topic, visit the page "High Spin and Low Spin".
Explanation: The first thing that should be done is calculate the oxidation number of "Mn". Since each \(\ce{NH3}\) has a negative one charge, and the overall charge is positive 2, then you can conclude that the Mn value will be positive 2. Just as before, this indicates that the electron configuration will be [Ar]d5. This shows that there are 5 electrons and that the molecule will be paramagnetic. It is known that \(\ce{NH3}\) is a strong field component of this complex, so that indicates a low spin as shown in the diagram. The diagram also indicates that the shape will be tetrahedral. This is due to the large crystal field splitting that is occurring.
Q24.13A
The following rates of reaction were obtained in three experiments with the reaction \(\ce{2NO(g) + Cl2(g) \rightarrow 2NOCl (g)}\).
| Expt | Initial \(\ce{[NO]}\), M | Initial \(\ce{[Cl2]}\), M | Initial Rate of Reaction, M s-1 |
|---|---|---|---|
| 1 | 0.0125 | 0.0260 | 2.23 x 10-5 |
| 2 | 0.0125 | 0.0510 | 4.50 x 10-5 |
| 3 | 0.0250 | 0.0260 | 9.05 x 10-5 |
What is the rate law of this reaction?
S24.13A
Explanation: As observed in the chart given, when the initial concentration of \(\ce{[NO]}\) is doubled, the rate of the reaction is also doubled. Also, when the initial concentration of \(\ce{[Cl2]}\) is doubled, the rate of the reaction is also doubled. This indicates that the reaction is first order. The rate law for the first order reaction would be k[A].
Q24.59C
The following statements about catalysis are not stated completely correct. What slight changes would you make to them?
(a) A catalyst is a substance that speeds up a chemical reaction but does not take part in the reaction.
(b) The function of a catalyst is to lower the activation energy allowed for a chemical reaction.
S24.59C
a. A catalyst is a substance that speeds up a chemical reaction but does not get consumed in the reaction.
Explanation: The part of this statement that should be changed is the phrase "does not take part" to "does not get consumed". The catalyst is taking part of the reaction by slowing it down and requiring a lower activation energy, but it will not be shown in the overall written reaction.
b. The function of a catalyst is to lower the activation energy needed for a chemical reaction.
Explanation: The part of the statement that should be changed is the phrase "allowed" to "needed". The activation energy is there so that the reaction is able to begin if it possess that much energy. The reaction needs the activation energy or else it would be spontaneous all the time.
Q25.27F
A wooden atlatl is discovered sitting atop a glacier in the Netherlands as the ice recedes. Since relative stratification is not an option in dating the ancient device, archaeologists use radiocarbon dating to reveal a decay rate of 15 dis h-1. A recent sample of freshly cut wood of the same type reveals a decay rate of 30 dis h-1. What is the age of the atlatl?
S25.27F
The problem asks us to determine the age of a recently exposed atlatl (a simple throwing device used to hurl spears or rocks with greater torque than that of the human arm) by using radiocarbon dating.
Knowing that the \(\mathrm{\textrm{half-life of Carbon-14} = 5730\, years}\), we can use these equations:
\[\mathrm{\lambda = \dfrac{0.693}{t_{1/2}}}\]
\[\mathrm{\ln\left(\dfrac{N_t}{N_0}\right) = - \lambda t}\]
Find the rate constant:
\[\mathrm{\lambda = \dfrac{0.0693}{57304} = 1.21\times10^{-4}}\]
Date the material:
Knowing that a recently cut material has a decay rate of 30 dis h-1, we can compare to the found atlatl.
\[\mathrm{\ln(15) - \ln(30) = -(1.21\times 10^{-4}\,y^{-1})(t)}\]
\[\mathrm{t = 5728.48\, y \sim 5700\, y}\]
The atlatl is approximately 5700 years old.
Explanation: The two formulas used at the beginning will help us determine the age of the wooden atlatl. These are equations that will either be given in the equation or on an equation sheet. The half life of carbon-14 will also be given to use in the first equation. The information given in the problem can simply be plugged into the equations to reveal that the atlatl is about 5700 years old.
Q21.1.3
Would you expect nonionizing or ionizing radiation to be more effective at treating cancer? Why?
S21.1.3
Ionizing radiation is higher in energy and causes greater tissue damage, so it is more likely to destroy cancerous cells.
Explanation: The greater tissue damage would cause a greater possibility of killing the cancerous cells, which would be the whole purpose of the radiation.
Q24.5.1
-
How many unpaired electrons are found in oxygen atoms ?
-
How many unpaired electrons are found in bromine atoms?
-
Indicate whether boron atoms are paramagnetic or diamagnetic.
-
Indicate whether F- ions are paramagnetic or diamagnetic.
-
Indicate whether Fe2+ ions are paramagnetic or diamagnetic.
S24.5.1
-
The O atom has 2s22p4 as the electron configuration. Therefore, O has 2 unpaired electrons.
-
The Br atom has 4s23d104p5 as the electron configuration. Therefore, Br has 1 unpaired electron.
- The B atom has 2s22p1 as the electron configuration. Because it has one unpaired electron, it is paramagnetic.
- The F- ion has 2s22p6 has the electron configuration. Because it has no unpaired electrons, it is diamagnetic.
- The Fe2+ ion has 3d6 has the electron configuration. Because it has 4 unpaired electrons, it is paramagnetic.
Explanation:
1. According to Hund's rule, electrons should not be paired right away, rather one electron should be placed at each possible electron location. When this is done, the oxygen has 2 unpaired electrons.
2. Using the same explanation as above, bromine will have 1 unpaired electron.

3-5. After the electron configuration is figured out, diamagnetic and paramagnetic is straight forward to figure out. Diamagnetic molecules contain no unpaired electrons; their orbitals are completely filled. Paramagnetic molecules contain unpaired electrons; their orbitals are not completely filled.

