Extra Credit 18
- Page ID
- 83411
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q19.5C
Determine \(\mathrm{\Delta G^\circ}\) for the following voltaic cell reactions:
- \(\mathrm{Pb^{2+}(aq) +Sn(s) \rightarrow Pb(s) +Sn^{2+}(aq)}\)
- \(\mathrm{O_2(g) +2H^+(aq) + 2F^-(aq) \rightarrow H_2O_2(aq) + F_2(g)}\)
- \(\mathrm{Br_2(l) +2Fe^{2+}(aq) \rightarrow 2Br^-(aq) + 2Fe^{3+}(aq)}\)
S19.5
Solution for a:
Step 1: Separate the reaction into its two half reactions
\[Sn {(s)} \rightarrow Sn^{2+}{(aq)}\]
\[Pb^{2+}{(aq)} \rightarrow Pb{(s)}\]
Step 2: Balance the half equation charges using e-
\[Sn{(s)} \rightarrow Sn^{2+}{(aq)}+2e^{-}\]
\[2e^{-}+Pb^{2+}{(aq)} \rightarrow Pb{(s)}\]
Step 3: From the balanced half reactions, we can conclude the number of moles of e- for use later in the calculation of ∆G. Determine the E° values using the standard reduction potentials, using the E° cell table. (For more information click here)
\[Sn{(s)} \rightarrow Sn^{2+}{(aq)}+2e^{-} \] \(E°=+0.137V\)
\[2e^{-}+Pb^{2+}{(aq)} \rightarrow Pb{(s)}\] \(E°=-0.125V\)
Step 4: Determine E°cell = E°reduction - E°oxidation (oxidation loses electrons, reduction gains electrons)
= -0.0125 - (0.137)
= -0.262 V
Step 5: Once E° cell has be calculated and the number of moles of electrons have been determined, we can use ∆G = -nFE°cell (where n is the number of moles of electrons transferred and F is faraday's constant).
= (-2 mol e-)(96458 C/mol e-)(-0.262 V)
∆G = 50544kJ
Solution for b:
Step 1: Separate the reaction into its two half reactions
\[2F^{-}{(aq)} \rightarrow F_{2}(g)\]
\[O_{2} (g)+ 2H^{+}{(aq)} \rightarrow H_{2}O{(aq)}\]
Step 2: Balance the half equations using O, H, and charge using e-
\[4F^{-}{(aq)} \rightarrow 2F_{2} (g)+4e^{-}\]
\[4e^{-}+O_{2} (g)+ 4H^{+}{(aq)} \rightarrow 2H_{2}O{(aq)}\]
Step 3: From the balanced half reactions, we can conclude the number of moles of e- for use later in the calculation of ∆G. Determine the E° values using the standard reduction potentials, using the E° cell table. (For more information click here)
\[4F^{-}{(aq)} \rightarrow 2F_{2} (g)+4e^{-}\] \(E°=-2.866V\)
\[4e^{-}+O{2 (g)}+ 4H^{+}{(aq)} \rightarrow 2H_{2}O{(aq)}\] \(E°=+1.229V\)
Step 4: Determine E°cell = E°reduction - E°oxidation (oxidation loses electrons, reduction gains electrons)
= 1.229 - (2.866)
= -1.637 V
Step 5: Once E° cell has be calculated and the number of moles of electrons have been determined, we can use ∆G = -nFE°cell (where n is the number of moles of electrons transferred and F is Faraday's constant).
= (-4 mol e-)(96458 C/mol e-)(-1.637 V)
∆G = 631606.98kJ
Solution for c:
Step 1: Separate the reaction into its two half reactions
\[2Fe^{2+}{(aq)} \rightarrow 2Fe^{3+}{(aq)}\]
\[Br_{2 (l)} \rightarrow 2Br^{-}{(aq)}\]
Step 2: Balance the half equation charges using e-
\[2Fe^{2+}{(aq)} \rightarrow 2Fe^{3+}{(aq)}+2e^{-}\]
\[2e^{-}+Br_{2 (l)} \rightarrow 2Br^{-}{(aq)}\]
Step 3: From the balanced half reactions, we can conclude the number of moles of e- for use later in the calculation of ∆G. Determine the E° values using the standard reduction potentials, using the E° cell table. (For more information, click here)
\[2Fe^{2+}{(aq)} \rightarrow 2Fe^{3+}{(aq)}+2e^{-}\] \(E°=-0.771V\)
\[2e^{-}+Br_{2 (l)} \rightarrow 2Br^{-}{(aq)}\] \(E°=+1.065V\)
Step 4: Determine E°cell = E°reduction - E°oxidation (oxidation loses electrons, reduction gains electrons)
= 1.065 - (0.771)
= 0.294 V
Step 5: Once E° cell has be calculated and the number of moles of electrons have been determined, we can use ∆G = -nFE°cell (where n is the number of moles of electrons transferred and F is faraday's constant).
= (-2 mol e-)(96458 C/mol e-)(1.836 V)
∆G = -56717.304 kJ
Q19.45C
The following voltaic cell is constructed:
\(\mathrm{Pb(s)||Pb^{2+}(satd\:PbI_2)||Pb^{2+}(0.100\:M)|Pb(s)}\). Given that the \(\mathrm{E_{cell} = 0.0567\: V}\), find the \(\mathrm{K_{sp}}\).
S19.45C
Set up the Nernst equation with the values appropriate for this equation. The moles of electrons transferred are 2. The \(\mathrm{E^\circ_{cell}}\) for this would be 0.
Thus, the Nernst equation sets up as \(\mathrm{0.0567 = E^{\circ}_{cell} - \dfrac{0.0592}{n}\times \log Q}\)
\(\mathrm{0.0567 = E^{\circ}_{cell} - \dfrac{0.0592}{n}\times \log \dfrac{x}{0.100}}\)
Solve for the value of x algebraically; this will yield an X value of 0.001215.
This value is not the answer; it is how much \(\ce{Pb^2+}\) needs to be saturated in the equation. To find the \(\mathrm{K_{sp}}\), note the stoichiometric values for \(\ce{[Pb]}\) and \(\mathrm{[I^-]^2}\). The \(\ce{Pb}\) and \(\ce{I}\) concentrations have been found. Now plug in your x value and \(\mathrm{K_{sp} = [Pb][I^-]^2}\)and \(\mathrm{K_{sp} =1.79 \times 10^{-9}}\).
For review on this topic, visit the page "Electrochemistry 4: The Nernst Equation".
Q21.3C
Name these complex compounds:
- \(\ce{[CoCl(H2O)2(NH3)3]I2}\)
- \(\ce{[CrBr2(CO)2(NO)2]+}\)
- \(\ce{K2[Fe(CN)4]}\)
- \(\ce{[CuI2BrCO]^2-}\)
- \(\ce{[Fe(ox)Cl2(H2O)]-}\)
S21.3C
- triamminediaquachlorocobalt(III) iodide
- dibromodicarbonyldinitrosylchromium(III) ion
- potassium tetracyanoferrate(II)
- bromocarbonyldiiodocuprate(I) ion
- aquadichlorooxalatoferrate(III) ion
For review on this topic, visit the page "Nomenclature of Coordination Complexes".
Q24.10A
At 65°, the half-life for the first-order decomposition of \(\ce{N2O5(g)}\) is 2.35 minutes.
\(\ce{N2O5(g) \rightarrow 2NO2(g) + \dfrac{1}{2}O2(g)}\)
If 1.00g of \(\ce{N2O5}\) is introduced into an evacuated 10L flask at 65°C,
- What is the initial partial pressure, in mmHg, of \(\ce{N2O5(g)}\)?
- What is the initial partial pressure, in mmHg, of \(\ce{N2O5(g)}\) after 2.35 minutes?
- What is the total gas pressure, in mmHg, after 2.35 minutes?
S24.10A
Step 1:
In order to find the initial partial pressure, we need to utilize the equation PV=nRT.
Moles of \(\ce{N2O5(g)}\) = \(\ce{1(g)}\) / \(\ce{108(g/mol)}\) = 0.009259 moles
\(\ce{P=}\) 0.009259 moles x 0.08206 L atm K-1mol-1 x 338 K / 10 L
\(\ce{P=}\) 0.025682 atm
0.025682 atm x 760 mmHg/atm =19.5181 mmHg
Step 2:
In order to find the specific partial pressure after 2.35 minutes, we utilize a rearranged versioon of the integrated rate law ln(2) = t1/2k. (For more information click here)
t1/2= 2.35 mins
ln(2)= 0.693
0.693/2.35 = k
k=0.294956
We then plug k into the equation ln(x)/inital partial pressure= -k x t1/2 for this specific equation.
ln(x)/19.5181= -0.294956 x 2.35
ln(x)/19.5181= -0.693147
x= 9.9.759 mmHg
This value is the initial partial pressure and the total gas pressure for this reaction after 2.35 minutes.
Q24.49A
Given the following graph:
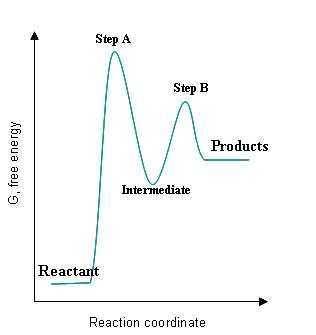
Please answer the following,
- How can you tell where the intermediate is on the graph?
- How can you tell where the transition state(s) is/are on the graph?
- How can you tell where the fastest step of the reaction is on the graph?
- How can you tell which step has the smallest rate constant?
- How can you tell whether the steps of the reaction are exothermic or endothermic?
- How can you tell whether the entire reaction is exothermic and endothermic?
S24.49A
- The intermediate is a local minimum and must be brought back up to a transition state before becoming the final product(s).
- The transition states, labeled Step A and Step B, are local maxima of the graph because they have reached the activation energy state needed to become intermediates/products.
- The fastest step of the reaction is the one with the smallest activation energy, which in this case would be Step B because the energy gap between the intermediate and the Step B transition step is much smaller than the gap between the original reactant and the Step A transition state.
- The smallest rate constant would correspond to the slowest reaction, which would correspond to the largest activation energy. In this case, Step A would have the smallest rate constant.
- The steps are exothermic if their intermediates/products are lower in energy than their reactants, and the steps are endothermic if their intermediates/products are higher in energy than their reactants. Step A and Step B are endothermic because their intermediates/products are higher in energy than their reactants.
- The overall reaction is exothermic if the initial reactant is higher in energy than the final products, and it is endothermic if the initial reactant is lower in energy than the final products. This reaction is endothermic because the reactant is lower in energy than the final products.
Q25.25E
Suppose that a sample of \(\ce{^{23}Mg}\) (\(\mathrm{\textrm{Half-life} = 11.32\, s}\)) has an activity 500 times the detectable limit. How long could experiments be run before radiation falls below detectable limits?
S25.25D
\(\mathrm{Half\: life\: of\: ^{23}Mg = 11.32\: seconds}\)
So first, to find the radioactive decay rate constant λ, we half to use the equation \(\mathrm{\lambda = \dfrac{0.693}{Half\: life}}\), and plug in what we've got:
\(\mathrm{\lambda = \dfrac{0.693}{11.32\, s} = 0.0612\, s^{-1}}\)
Then, since our sample has an activity 500 times the detectable limit, we plug 500 and our decay rate constant into the equation below and solve for t (the amount of time the experiment can be run):
\(\mathrm{\ln\left(\dfrac{1}{500}\right) = -0.0612\, s^{-1} (t)}\)
\(\mathrm{t= 101.5\: seconds}\)
The experiments could be run for 101.5 seconds before radiation falls below detectable limits.
To review half life and activity, visit the page "Radioactive Decay Rates".
Q18.5
For each of the following reactions: 1) find \(\mathrm{E^\circ}\) (in volts) and 2) determine whether the reaction is spontaneous under standard conditions.
- the reaction between iron and iron(III) ions to give iron(II) ions.
- the following cell: \(\mathrm{ I^- \, |\, I_2 \,||\, Zn^{2+} \,|\, Zn}\)
S18.5
Solution for a:
Step 1: Separate the reaction into its two half reactions
\[Fe_{(s)} \rightarrow Fe^{2+}_{(aq)}\]
\[Fe^{3+}_{(aq)} \rightarrow Fe^{2+}_{(aq)}\]
Step 2: Balance the half equation charges using e-
\[Fe_{(s)} \rightarrow Fe^{2+}_{(aq)}+2e^{-}\]
\[2e^{-}+2Fe^{3+}_{(aq)} \rightarrow 2Fe^{2+}_{(aq)}\]
Step 3: From the balanced half reactions, we can conclude the number of moles of e- for use later in the calculation of ∆G. Determine the E° values using the standard reduction potentials, using the E° cell table. (For more information, click here)
\[Fe_{(s)} \rightarrow Fe^{2+}_{(aq)}+2e^{-}\] Eo = -0.440V
\[2e^{-}+2Fe^{3+}_{(aq)} \rightarrow 2Fe^{2+}_{(aq)}\] Eo = +0.771V
Step 4: Determine E°cell = E°reduction - E°oxidation (oxidation loses electrons, reduction gains electrons)
Eocell = 0.771V - (0.440V)
Eocell= +0.331 V
Step 5: Once E° cell has be calculated and the number of moles of electrons have been determined, we can use ∆G = -nFE°cell (where n is the number of moles of electrons transferred and F is faraday's constant).
= (-2 mol e-)(96458 C/mol e-)(0.331 V)
∆G = -63855.2 kJ
Step 6: Now that we know ∆G, we can determine the spontaneity of the reaction. When ∆G is positive, the reaction is non- spontaneous and when it is negative, it is spontaneous. Since ∆G is negative for this reaction, it is spontaneous under standard conditions.
Solution for b:
Step 1: Separate the reaction into its two half reactions
\[2I^{-} \rightarrow I_{2}\]
\[Zn^{2+} \rightarrow Zn\]
Step 2: Balance the half equation charges using e-
\[2I^{-} \rightarrow I_{2}+2e^{-}\]
\[2e^{-}+Zn^{2+} \rightarrow Zn\]
Step 3: From the balanced half reactions, we can conclude the number of moles of e- for use later in the calculation of ∆G. Determine the E° values using the standard reduction potentials, using the E° cell table. (For more information click here)
\[2I^{-} \rightarrow I_{2}+2e^{-}\] -Eo = +0.535V
\[2e^{-}+Zn^{2+} \rightarrow Zn\] Eo = -0.763V
Step 4: Determine E°cell = E°reduction - E°oxidation (oxidation loses electrons, reduction gains electrons)
Eocell = -0.763V - (0.535V)
Eocell= -1.298 V
Step 5: Once E° cell has be calculated and the number of moles of electrons have been determined, we can use ∆G = -nFE°cell (where n is the number of moles of electrons transferred and F is faraday's constant).
= (-2 mol e-)(96458 C/mol e-)(-1.298 V)
∆G = 250405kJ
Step 6: Now that we know ∆G, we can determine the spontaneity of the reaction. When ∆G is positive, the reaction is non- spontaneous and when it is negative, it is spontaneous. Since ∆G is negative for this reaction, it is non- spontaneous under standard conditions.
For more examples and explanations (For more information click here).
Q21.4.2
If a sample of one isotope undergoes more disintegrations per second than the same number of atoms of another isotope, how do their half-lives compare?
S21.4.2
An isotope that undergoes more disintegrations per second will have a faster reaction time than an isotope with the same number of atoms undergoing less disintegrations per second (because more is happening in a period of time). Because of this, the faster reaction (more disintegrations) will have a shorter half-life and the slower reaction (less disintegrations) will have a longer half-life, as it will take more time for the slower reaction to react.
This can also be seen mathematically as follows:
\[t_{1/2} = \frac{0.693}{k}\]
Clearly, the half life is inversely proportional to k. And k is related to activity (disintegrations per second) as follows:
\[A=kN\]
So, higher the disintigrations per second or (activity), higher the value of k and smaller the value of half life.

