Extra Credit 43
- Page ID
- 82752
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.2
Aluminum (E∘ Al/Al=−2.07V) is more easily oxidized than iron (E∘ Fe3+/Fe=−0.477V) and yet when both are exposed to the environment, untreated aluminum has very good corrosion resistance while the corrosion resistance of untreated iron is poor. Explain this observation.
A17.6.2
Corrosion is the oxidation of a material due to its reaction to the surrounding environment. This causes a rough layer to form on the metal's surface.
Aluminum has a very good corrosion resistance because it forms an outer layer of oxide. This helps prevent oxygen from reaching the aluminum with ease, therefore preventing corrosion and further oxidation. While considering the characteristics of each metal it is true that aluminum forms an immediate oxide layer and then it stops reacting. In the case of iron, it keeps reacting to form multiple oxide layers that flake off. This can be seen on the outside of a nail forming a red layer when exposed to oxygen and easily flakes off. Therefore, iron will corrode while aluminum stays protected.
Q12.3.6
Regular flights of supersonic aircraft in the stratosphere are of concern because such aircraft produce nitric oxide, NO, as a byproduct in the exhaust of their engines. Nitric oxide reacts with ozone, and it has been suggested that this could contribute to depletion of the ozone layer. The reaction NO+O3⟶NO2+O2 is first order with respect to both NO and O3 with a rate constant of 2.20 × 107 L/mol/s. What is the instantaneous rate of disappearance of NO when [NO] = 3.3 × 10−6 M and [O3] = 5.9 × 10−7 M?
A12.3.6
The instantaneous rate of reaction is the rate of reaction at any instant of time during the reaction.
First we look at the given values and what we are asked for in the question. We know that rate=k[A]a[B]b and we are looking for the (instantaneous) rate. The value 2.2 x 107 mol/L/s is our k and the values [NO] = 3.3 × 10−6 M and [O3] = 5.9 × 10−7 M are our concentrations or [A] and [B]. Lastly we are told that the reaction is first order in respect to both NO and O3 so we know that our values of a and b are one.
Second, we look at the given our given reaction of NO+O3⟶NO2+O2 and set up the rate law -k[NO]1[O3]1 . It is important to notice that the arrow is only going forward unlike in equilibrium the arrow is going forwards and backwards, so in this reaction the left side is the reactant and the right side is the product. In problems regarding rates in kinetics of forward arrow single replacement problem we only consider the reactants. Next we plug in the values to get our overall reaction rate for the reaction.
rate = -k[NO][O3]
rate= -(2.2 x 107 L/mol/s)( 3.3 × 10−6M )1( 5.9 × 10−7M)1
we then get the solution -4.28 x 10-5 mol/L/s.
the rate here is negative and rates can only be positive. We know that this question is asking for the instantaneous rate of disappearance of NO so we must consider the rate of -(-4.28 x 10-5mol/L/s) to get the value 4.28 x 10-5 mol/L/s as our instantaneous rate of disappearance of NO.
Q12.5.15
The hydrolysis of the sugar sucrose to the sugars glucose and fructose,
C12H22O11+H2O⟶C6H12O6+C6H12O6
follows a first-order rate equation for the disappearance of sucrose: Rate = k[C12H22O11] (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules.)
- In neutral solution, k = 2.1 × 10−11 s−1 at 27 °C and 8.5 × 10−11 s−1 at 37 °C. Determine the activation energy, the frequency factor, and the rate constant for this equation at 47 °C (assuming the kinetics remain consistent with the Arrhenius equation at this temperature).
- When a solution of sucrose with an initial concentration of 0.150 M reaches equilibrium, the concentration of sucrose is 1.65 × 10−7 M. How long will it take the solution to reach equilibrium at 27 °C in the absence of a catalyst? Because the concentration of sucrose at equilibrium is so low, assume that the reaction is irreversible.
- Why does assuming that the reaction is irreversible simplify the calculation in part (b)?
A12.5.15
The activation energy of a reaction is the minimum energy required by the reactants to undergo a collision and cross the energy barrier to move forward and form the products.
1. First we evaluate our given values and see which equations they might fit into. The variation of the Arrhenius equation ln \((\frac{k1}{k2})\) =\((\frac{-Ea}{R})\)\( ((\frac{1}{T2})\) - \((\frac{1}{T1})\) )is used since we are given two different temperatures and two two rate constants corresponding to one solution. Plug in the values and solve.
ln\((\frac{8.5 E-11}{2.1 E-11})\) = \((\frac{-Ea}{R})\) [ \((\frac{1}{300})\) - \((\frac{1}{310})\) ]
1.40 1/s= \((\frac{-Ea}{R})\) (-1.075 E-4 K)
\((\frac{1.40 1/s}{-1.075 E-4 K})\) =\((\frac{-Ea}{R})\)
(-8.314J/mol*K)(-1.30E4 K/s) = Ea
remember it is wanted in kJ not J so we must convert our answer.
108277.3 / 1000 =108 kJ
Ea = 108 kJ
now that we have the Ea we use the Arrhenius equation to find A at one of our given temperatures and rate constants to get 1.3 x 108
k = Ae\((\frac{-Ea}{Rt})\)
8.5 × E-11 s−1 = Ae\((\frac{108kj}{(8.314J/mol*K)(310K)\)
\((\frac{8.5 E-11}{6.54 E-19})\) = A
Last we solve for our rate constant by plugging in all the values and our temperature 47 to get 3.05 x 10-10 for our overall k
k = (1.3 x 108)e\((\frac{108kj}{(8.314J/mol*K)(320K)\)
k = 3.05 x 10-10
2. Using the first order integrated rate law equation ln[A]= ln[Ao]-kt. A is our concentration of sucrose, Ao is our initial concentration. K at 27 °C is given from question 1 at 2.1 × 10−11 s−1. You must plug in your values and solve for the amount of time that it takes to reach equilibrium at 27 °C. Don't forget log rules and converting Celsius to Kelvin.
ln[1.65 E-7M] = ln[ 0.150 M] -(2.1 E-11 1/s)t
ln\((\frac{ 1.65 E-7 }{0.150})\) = -2.1 × E-11 1/s (t)
6.53 E-11 s = t
However this answer is in seconds and we want it in hours (or days).
6.53 x E-11 s =1min/60secs =1hr/ 60mins = 1.8x 108 hours
or 1.8x 108 hours =1 day/ 24hours = 7.6 × 106 days.
3. Assuming that the reaction is irreversible simplifies the calculation because we do not have reconsider any reactant reacting in the reverse direction to return to its original state.
Q21.4.10
Predict by what mode(s) of spontaneous radioactive decay each of the following unstable isotopes might proceed:
- He6
- \(\ce{^{60}_{30}Zn}\)
- \(\ce{^{235}_{91}Pa}\)
- 18F
- 129Ba
- 237Pu
A21.4.10
Radioactivity is the process in which particles are emitted from the unstable nucleus to form a stable nucleus.
A positron is a particle that is similar to a electron, but it has an opposite charge.
Beta decay is when a neutron in the nucleus is converted to a proton and an electron is emitted from an atom.
If the atomic mass of the element given is higher than the respective atomic mass on the Periodic Table, it is likely to be BETA DECAY.( (\(\ce{^{0}_{-1}e}\) )
If the atomic mass of the element given is lower than the respective atomic mass on the Periodic Table, it is likely to be POSITRON EMISSION. ( (\(\ce{^{0}_{1}e}\) )
Remember that the atomic number and number of protons must be equal on both sides.
1. He6⟶ \(\ce{^{0}_{-1}e}\) + \(\ce{^{6}_{3}Li}\)
2. \(\ce{^{60}_{30}Zn}\) ⟶ \(\ce{^{0}_{1}e}\) + \(\ce{^{60}_{29}Cu}\)
3. \(\ce{^{235}_{91}Pa}\) ⟶ \(\ce{^{0}_{-1}e}\) + \(\ce{^{235}_{92}U}\)
4. \(\ce{^{241}_{93}Np}\) \(\ce{^{0}_{-1}e}\) + \(\ce{^{241}_{94}Pu}\)
5. 18F⟶ \(\ce{^{0}_{1}e}\) + \(\ce{^{18}_{8}O}\)
6. 129Ba ⟶ \(\ce{^{0}_{1}e}\) + \(\ce{^{129}_{55}Cs}\)
7. 237Pu ⟶ \(\ce{^{0}_{-1}e}\) + \(\ce{^{237}_{92}U}\)
Q20.2.14
Copper metal readily dissolves in dilute aqueous nitric acid to form blue Cu2+(aq) and nitric oxide gas.
- What has been oxidized? What has been reduced?
- Balance the chemical equation.
A20.2.14
Oxidation is the loss of electrons, while reduction is the gain of electrons. An easy way to memorize this is OIL RIG (oxidation is loss and reduction is gain)
1. To figure out what is reduced or oxidized we must first write the original chemical equation then we divide it into half reactions.
We conclude the overall reaction from the given information is Cu(s)+ HNO3(aq)⟶ Cu2+(aq)+NO(g).
Our first half reaction is: Cu(s) → Cu2+(aq) + 2e-
From Cu to Cu2+ it is becoming more positve.As shown in the equation, it has to loose electrons on the left and give electrons on the product side to keep the reaction neutral. Therefore Cu is being oxidized.
Our second half reaction is: HNO3(aq) + 3H+(aq) + 3e- → NO(g) + 2H2O(l)
Finding which side needs to have electrons added to it requires a little more attention. On the right side the overall charge is +3 and the right side is on the right side the charge is 0. Therefore we add electrons to the left side. Since we are gaining electrons on the left side we can conclude that nitrogen is being Reduced.
2. We must have the same electrons in both equations so we multiply the first equation by 3 and the second one by 2
3[Cu(s) → Cu2+(aq) + 2e-]
2[HNO3(aq) + 3H+(aq) + 3e- → NO(g) + 2H2O(l)]
we must multiply everything by the factor of 2 or 3 respectively to get
3Cu(s) → 3Cu2+(aq) + 6e-
2HNO3(aq) + 6H+(aq) + 6e- → 2NO(g) + 4H2O(l)
now we can cancel the electrons and get the combine the reactions to get the overall reaction in acidic conditions as
3Cu(s)+2HNO3(aq) + 6H+(aq) →3Cu2++ 2NO(g) + 4H2O(l)

Q20.5.9
Concentration cells contain the same species in solution in two different compartments. Explain what produces a voltage in a concentration cell. When does V = 0 in such a cell?
A20.5.9
A voltage is produced in a concentration cell when one solutions concentration is lower than the others. For example, the lower concentration(1) solution will transfer electrons to the higher concentration solution (2) to plate out the metal in the solution(2) to lower the concentration. The higher concentration solution (2)decreases to match the concentration of the lower solution (1) until the redox potential E=0, so therefore V =0.
Q24.6.5
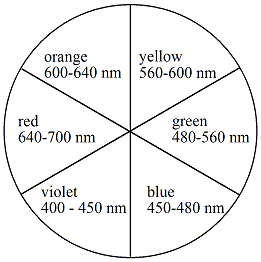
How can CFT explain the color of a transition-metal complex?
A24.6.5
When metals are not bonded to anything they are degenerate with the same energy. The color of a transition metal depends on the amount of electrons they have in their d-orbitals. When bonding with ligands these degenerate energies are effected this is because of the Crystal Field Theory. Partially filled d-orbitals have high splitting energies that produce frequencies that lie in the visible light range.
depends on splitting from ligand. high low spin, smaller wavelength larger energy more color. related by \((\frac{hc}{ λ })\)