Extra Credit 4
- Page ID
- 82748
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Q17.1.4
For each of the following unbalanced half-reactions, determine whether an oxidation or reduction is occurring.
⟶
⟶
⟶
⟶NO
S17.1.4
(a) oxidation is occurring because the oxidation number of Cl goes from -1 to 0. Oxidation is defined as an increase in oxidation number and as loss of electrons.
(b) oxidation, the oxidation number of Mn on the left side is +2(the given charge) and on the right side oxygen has an oxidation number of -2 and there are to of them so that makes -4 charge, so Mn must have an oxidation number of +4. There is in an increase n oxidation number therefore oxidation is occuring
(c) oxidation, the oxidation number of H increase from 0 to +1
(d) reduction, the oxidation number of N decrease from +5 (O have and oxidation of number of -2 and there are there of them so that -6 and the ion has a -1 charge for N must have and oxidation number of +5)
Q19.1.2
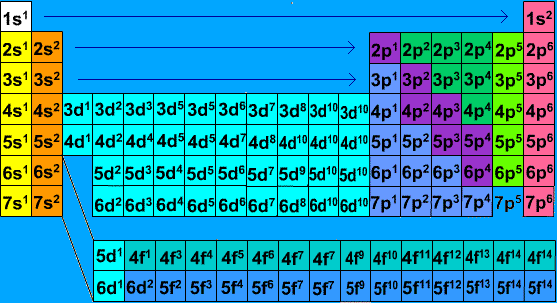
Write the electron configurations for each of the following elements and its ions:
- Ti
- Ti2+
- Ti3+
- Ti4+
S19.1.2
- [Ar]
using the chart below you can locate the element Ti. And with Cations you want to remove the amount of electron equivalent to the charge. You want to remove electrons from the 4s orbital first and then from the 3d orbital.
Q19.2.4
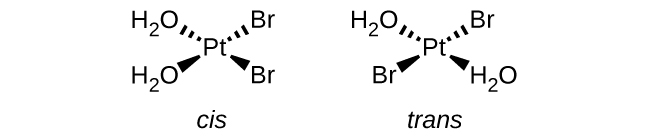
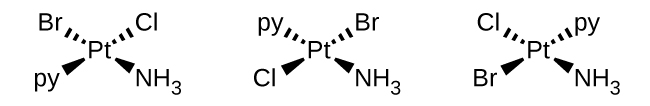
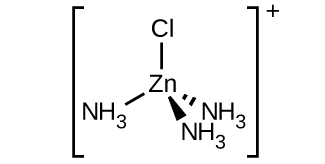
Sketch the structures of the following complexes. Indicate any cis, trans, and optical isomers.
- [Pt(H2O)2Br2] (square planar)
- [Pt(NH3)(py)(Cl)(Br)] (square planar, py = pyridine, C5H5N)
- [Zn(NH3)3Cl]+ (tetrahedral)
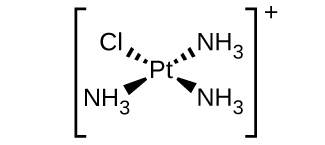
- [Pt(NH3)3Cl]+ (square planar)
- [Ni(H2O)4Cl2]
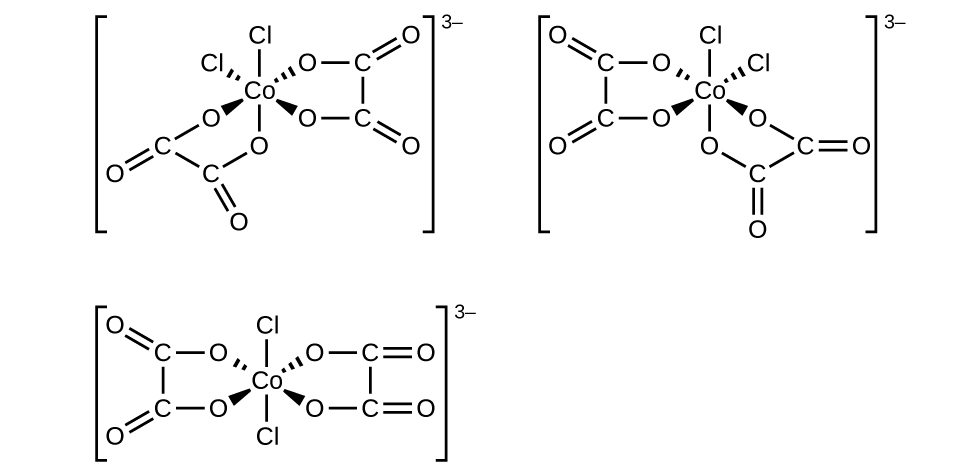
- [Co(C2O4)2Cl2]3− (note that C2O2−4C2O42− is the bidentate oxalate ion, −O2CCO−2−O2CCO2−)
S19.2.4
a. [Pt(H2O)2Br2]:
b. [Pt(NH3)(py)(Cl)(Br)]:
c. [Zn(NH3)3Cl]+ :
d. [Pt(NH3)3Cl]+ :
e. [Ni(H2O)4Cl2]:
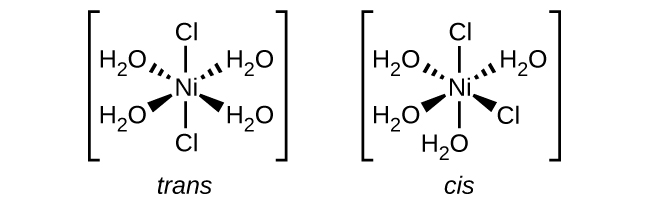
f. [Co(C2O4)2Cl2]3−:
Q12.3.17
Hydrogen reacts with nitrogen monoxide to form dinitrogen monoxide (laughing gas) according to the equation:
\[\ce{H2}(g)+\ce{2NO}(g)⟶\ce{N2O}(g)+\ce{H2O}(g)]
Determine the rate equation, the rate constant, and the orders with respect to each reactant from the following data:
| [NO] (M) | 0.30 | 0.60 | 0.60 |
|---|---|---|---|
| [H2] (M) | 0.35 | 0.35 | 0.70 |
| Rate (mol/L/s) | 2.835 × 10−3 | 1.134 × 10−2 |
2.268 × 10−2 |
Q12.6.8
Nitrogen(II) oxide, NO, reacts with hydrogen, H2, according to the following equation:
What would the rate law be if the mechanism for this reaction were:
S12.6.8
The rate law always equals the slow step.
Q21.4.20
What is the age of mummified primate skin that contains 8.25% of the original quantity of ?
S21.4.20
First we want to use the equation and use the fact that we know the half life of
to be 5730 years.
Nest we plug this into the first order atomic decay equation: because we know
= .0001209 and we can assume the initial amount to be 100 (
) and 8.25 (
) to be the amount remaining after said time because in the problem they tell us 8.25% is left which can equal 8.25/100. With this we are left to solve for t. When you plug everything in you get that t is about 20,629 years.
Q20.3.8
![]()
Consider the following spontaneous redox reaction: NO3−(aq) + H+(aq) + SO32−(aq) → SO42−(aq) + HNO2(aq).
- Write the two half-reactions for this overall reaction.
- If the reaction is carried out in a galvanic cell using an inert electrode in each compartment, which electrode corresponds to which half-reaction?
- Which electrode is negatively charged, and which is positively charged?
S20.3.8
a. (reduction);
(oxidation)
b. The cathode corresponds with the first half-equation, while the anode corresponds with the second half-reaction. Reduction always occurs with the Cathode (both consonants) and Oxidation always occurs with the Anode (both vowels).
c. Cathode is considered positive for a galvanic cell because electron flow is towards the cathode, while anode is considered negative.
Q20.5.19
![]()
Potentiometric titrations are an efficient method for determining the endpoint of a redox titration. In such a titration, the potential of the solution is monitored as measured volumes of an oxidant or a reductant are added. Data for a typical titration, the potentiometric titration of Fe(II) with a 0.1 M solution of Ce(IV), are given in the following table. The starting potential has been arbitrarily set equal to zero because it is the change in potential with the addition of the oxidant that is important.
| Titrant (mL) | E (mV) |
|---|---|
| 2.00 | 50 |
| 6.00 | 100 |
| 9.00 | 255 |
| 10.00 | 960 |
| 11.00 | 1325 |
| 12.00 | 1625 |
| 14.00 | 1875 |
- Write the balanced chemical equation for the oxidation of Fe2+ by Ce4+.
- Plot the data and then locate the endpoint.
- How many millimoles of Fe2+ did the solution being titrated originally contain?
S20.5.19
1.
2.

The end point is is about at (10,1000), you can tell this because that is when the graphs slop is increasing close to a vertical line. The endpoint of a titration is when the moles of reactant exceed the moles of what is being titrated.
3. .1Mx10mL= 1mmol
You take the the molarity which in units of Mols/Liter and multiply by the mL of the endpoint which was 10mL to get millimoles.

