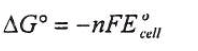Extra Credit 39
- Page ID
- 82747
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.5.7
Explain what happens to battery voltage as a battery is used, in terms of the Nernst equation.
S17.5.7
A battery are self contained, portable galvanic cells. This means the redox reactions inside the battery cell is what drives the flow of electrons which creates electricity or electrical current. When the battery voltage is used, the voltage of the system drops which means the system is spontaneously approaching equilibrium. This reaction is a spontaneous reaction so K >> 1. As the reaction proceeds, the Q will approach K which means the cell potential will drop. So as the reaction proceeds it will change Q. Another way of thinking about this is that the redox equation is approaching equilibrium and once it reaches equilibrium, the Eocell reaches = 0 and so does the delta G = 0. When the battery reaches equilibrium, the battery is considered "dead".
Recall the Nerst equation
Batteries typically have 2 or more electrochemical cells. They use chemical energy and convert it into electrical energy. To explain what happens to the battery voltage as the battery is used, we can look at the Nernst equation. Ecell refers to the cell potential while Eocell refers to the standard cell potential. R is a gas constant, T is the temperatures, n is the number of moles, F is the Faraday's constant, and Q is the ratio of molar concentrations of product to reactant.
We can look at the overall equation of an alkaline battery

Under the nernst equation, at equilibrium Q = K and Eocell = 0 , we can also assume that the reaction occurs at 25oC so the nernst equation will be
Ecell = Eocell - (.0591/n) log Q
since 2e- were in the system of alkaline battery we can substitute 2 for n
Ecell = 0 - (.0591/2) log Q ; Q in alkaline battery is [ZnO(s)][MnO3(s)] / [MnO2(s)]2 [Zn(S)]
As the reaction proceeds, the products concentration will be much higher than the reactant which means Q will be high. This means Ecell will eventually be 0 since 0 minus a really small number will equal 0.
Ecell = 0V
When Ecell reaches 0, the battery is "dead" and is no longer spontaneous. So in conclusion as the battery is being used, the voltage drops as reactant gets converted to products and eventually the cell potential will reach 0 and that is when the battery reaches equilibrium.
Phase II:
Addition to the stated above: As battery is being used, the battery voltage will decrease and eventually go dead as a result of either reagents in the battery being used up or redox reactions' byproducts accumulate and interfere with the reaction. As the redox reaction proceed to equilibrium, the value of Q will approach K, therefore if we combine the equations for K, "E°cell = (RT/nF)lnK " and Q, "Ecell = E°cell - (RT/nF)lnQ, we will get Ecell=(RT/nF)ln(K/Q). From the overall equation, we can see that when K/Q approaches 0, the cell potential will also approached 0, thus leading to a dead cell.
Q12.3.2
Doubling the concentration of a reactant increases the rate of a reaction four times. With this knowledge, answer the following questions:
- What is the order of the reaction with respect to that reactant?
- Tripling the concentration of a different reactant increases the rate of a reaction three times. What is the order of the reaction with respect to that reactant?
S12.3.2
1. Step 1: the rate of the reaction increases 4 times as much as the reactant doubles. Note Q = the concentration of the reactant. K = the rate constant. m= the order of reaction
4 x rate = K[2Q]m
Next can substitute the "rate" with K[Q]m
4 x K[Q]m = K[2Q]m
Take out the 2
4 x K[Q]m = 2mK[Q]m
Now we can cancel out the common terms.
4= 2m
Solve for m by taking the log of both sides.
log(4)= m log (2)
m= log(4) / log (2)
m = 2 so the reaction order is 2
2. Tripling the concentration of a different reactant increases the rate of a reaction three times. What is the order of the reaction with respect to that reactant?
Step 1: 3 times the concentration = 3 times the rate
3 x rate = k [3Q]m
Step 2: We can substitute k [Q]m as the "rate"
3 x k [Q]m= k [3Q]m
Step 3: Take out the 3 so that we can get common terms
3 x k [Q]m= 3m k[Q]m
Step 4: divide by common terms.
3 = 3m
Step 5: Take the log of both sides and find m
log (3) = m log (3)
m= log (3) - log (3) = 1
Reaction order is 1
Phase II:
k is the rate constant, [C] is the reactant's concentration, and n is the reaction order.
rate1=k[C1]n
rate2=k[C2]n
If rate2=4 x rate1 If rate2=3 x rate1
k[C2]n = 4 x k[C1]n k[C2]n = 3 x k[C1]n
([C2]/[C1])2=4 ([C2]/[C1])2=3
and [C2] = 2 x [C1] and [C2] = 3 x [C1]
(2 x [C1]/[C1])2=4 (3 x [C1]/[C1])2=3
2n = 4 3n = 3
n = 2 n = 1
Q12.5.11
An elevated level of the enzyme alkaline phosphatase (ALP) in the serum is an indication of possible liver or bone disorder. The level of serum ALP is so low that it is very difficult to measure directly. However, ALP catalyzes a number of reactions, and its relative concentration can be determined by measuring the rate of one of these reactions under controlled conditions. One such reaction is the conversion of p-nitrophenyl phosphate (PNPP) to p-nitrophenoxide ion (PNP) and phosphate ion. Control of temperature during the test is very important; the rate of the reaction increases 1.47 times if the temperature changes from 30 °C to 37 °C. What is the activation energy for the ALP–catalyzed conversion of PNPP to PNP and phosphate?
S12.5.11
We now that ALP catallayzes reaction between PNPP → PNP. To calculate the activation energy we have to use the equation.

We know that the rate of reaction of k2 = 1.47
rate of reaction of k1= 1
T1 = 30oC convert it to kelvins = 303K
T2 = 37oC convert it to kelvins = 310K
R = 8.314 J/mol x K
Step 1: substitute the values into the above equation and convert the activation energy to kJ/mol
ln (1.47/1) = Ea / (8.314 J/mol x K) [ (1/303K) - (1/310K) ]
=42983 J/mol x 1kJ/1000J
= 43.0 kJ/mol
Q21.4.6
Explain how unstable heavy nuclides (atomic number > 83) may decompose to form nuclides of greater stability (a) if they are below the band of stability and (b) if they are above the band of stability.
S21.4.6
The belt of stability is a region where the number of neutrons and protons form stable nuclei.
A. If a nuclei is below the band of stability and the element has a atomic number greater than 83, then it must emit a positron also known as electron capture to regain stability. This is because elements with atomic number greater than 83 have more protons compared to the number of neutrons.
Positron: It is often referred to as an antiparticle which is represented as 
B If you are above the band of stability, this means your neutron to proton ratio is too high so you want to decrease the number neutrons. To do so most isotopes will undergo beta decay.
Beta decay: This occurs when the neutron converts to a proton and a electron and the electron emits out of the atom.
Phase II:
The Band / Belt of Stability helps determine the stability of the isotope. The radionuclides that lie in the upper right end of the band of stability decay by alpha decay, below by position emission, and above by beta emissions. In addition, the stability of an element can also determine base on the number of nucleons (more likely to be stable if even), the protons and neutrons 's magic numbers, and the neutron/proton ratio.
Q20.2.10
Balance each redox reaction under the conditions indicated.
- MnO4−(aq) + S2O32−(aq) → Mn2+(aq) + SO42−(aq); acidic solution
- Fe2+(aq) + Cr2O72−(aq) → Fe3+(aq) + Cr3+(aq); acidic solution
- Fe(s) + CrO42−(aq) → Fe2O3(s) + Cr2O3(s); basic solution
- Cl2(aq) → ClO3−(aq) + Cl−(aq); acidic solution
- CO32−(aq) + N2H4(aq) → CO(g) + N2(g); basic solution
S20.2.10
In order to determine whether each equation is a reduction or oxidation equation we can determine the oxidation state of each element and see which element increases or decreases in oxidation state. If it increased in oxidation state then it went through oxidation, but if it decreased in oxidation state then it went through reduction. After identifying the oxidation and reduction half-reactions, you need to balance the half-reactions. To balance the half reactions you must count up the number of atoms of each element there are on each side of the equation. Balance the number of atoms so that both sides have the same number of atoms per element. Remember to always balance the hydrogens and oxygens last. If you need to balance the oxygens you can add water (H2O). Add H+ to balance out the hydrogens. Lastly make sure the charge is the same on both sides and you can add e- to balance the charge so that both sides are equal. (If a reaction is in acidic solution then use H+ to balance the hydrogens, but if it's in a basic solution then use OH- to neutralize the hydrogens. Keep in mind that when H+ and OH- combine together it forms H2O.) Once the charges are balance, multiple each equation by a factor so that the electrons cancel out. This maintains charge neutrality within the overall reaction equation. Don't forget to eliminate common species on both sides so that the overall reaction contains only the minimum amount of each species.
-
MnO4−(aq) + S2O32−(aq) → Mn2+(aq) + SO42−(aq); acidic solution
Step 1: First we need to assign oxidation numbers to see which species reduced or oxidized.
MnO4−(aq): the overall charge of the species is 1- and each O is 2- so together they are 4(-2)=8, therefore, the oxidation state of Mn is 7+.
S2O32−(aq): the charge of all the oxygens is 3 (-2) = -6 and the overall charge of the species is -2. So S = +2 since there are 2 of them so 2 (+2) = 4 which gives the species an overall charge of 2-
SO42−(aq): 4(-2) from the oxygen = -8. The overall charge of the species = -2 so the S has to be +6
Based on the oxidation numbers, we know that MnO4− is reduced and S2O32− was oxidized
Step 2: Determine the oxidation and reduction half reactions
Oxidation: S2O32−(aq)→SO42−(aq)
Reduction: MnO4−(aq)→ Mn2+(aq)
Step 3: Balance each half reaction. Use H2O to balance the oxygens and H+ to balance the H.
Oxidation: S2O32−+ 5H2O(aq)→2SO42−(aq) + 10H+(aq)+8e-
Reduction: MnO4−(aq)+ 8H+(aq) + 5e-→ Mn2+(aq) + 4H2O(aq)
Step 4: multiple the oxidation reaction by a factor of 5 and the reduction reaction by a factor of 8
Oxidation: (S2O32−+ 5H2O(aq)→2SO42−(aq) + 10H++8e-) X 5
Reduction: (MnO4−(aq)+ 8H+ (aq)+ 5e-→ Mn2+(aq) + 4H2O(aq) ) X 8
-----------------------------------------------------------------------------------
Oxidation: 5S2O32−+ 25H2O(aq)→10SO42−(aq) + 50H+(aq)+40e-
Reduction: 8MnO4−(aq)+ 64H+ (aq)+ 40e-→ 8Mn2+(aq) + 32H2O(aq)
Step 5: Cancel out the electrons and the species and write the overall equation
Overall reaction: 5S2O32−(aq)+8MnO4−(aq)+14H+(aq)→10SO42−(aq) + 8Mn2+(aq) +7H2O(l)
2. Fe2+(aq) + Cr2O72−(aq) → Fe3+(aq) + Cr3+(aq); acidic solution
Step 1: Assign oxidation numbers to each species
Fe2+(aq) became Fe3+(aq) which means it went through oxidation
Cr in Cr2O72−(aq) has a oxidation state of -2 = 7(-2) + x , x = 12-->Cr = 6+. It formed Cr3+(aq) which means it went through reduction since it decrease in oxidation state
Step 2: Determine the reduction/oxidation half-reactions
Reduction: Cr2O72−(aq)→Cr3+(aq)
Oxidation: Fe2+(aq) → Fe3+(aq)
Step 3: Balance half reactions
Reduction: Cr2O72−(aq) + 14 H+(aq) + 6e-→2Cr3+(aq) + 7H2O(aq)
Oxidation: (Fe2+(aq) → Fe3+(aq) +e-) x 6
Overall reaction: Cr2O72−(aq) + 14 H+(aq) + 6Fe2+(aq)→6Fe3+(aq) + 2Cr3+(aq) + 7H2O(aq)
3.Fe(s) + CrO42−(aq) → Fe2O3(s) + Cr2O3(s); basic solution
Step 1: Assign oxidation numbers to each species
Fe in Fe2O3(s) has an oxidation state of +3 which means it went through oxidation.
Cr in CrO42−(aq) +6 but in Cr2O3(s) the oxidation state of Cr is +3 which means Cr went through reduction.
Step 2: Determine the reduction/oxidation half-reaction
Reduction: Fe(s) → Fe2O3(s)
Oxidation: CrO42−(aq) → Cr2O3(s)
Step 3: Balance the redox half-reactions
Reduction: 2Fe(s) + 3H2O(aq) → Fe2O3(s) + 6H+(aq) + 6e-
Oxidation: 2CrO42−(aq) + 10H+(aq) + 6e-→ Cr2O3(s) + 5H2O(aq)
step 4: Cancel out the electrons and other species. Add OH- to both sides to neutralize the H+
Reduction: 2Fe(s) → Fe2O3(s)
Oxidation: 2CrO42−(aq) + 4H++ 4OH- (aq)→ Cr2O3(s) + 2H2O(aq)+ 4OH-(aq)
Step 5: Cancel out the H2O(aq) and write the overall reaction
Overall reaction: 2Fe(s)+2CrO42−(aq) +2H2O(aq)→ Cr2O3(s) + Fe2O3(s)+ 4OH-(aq)
4. Cl2(aq) → ClO3−(aq) + Cl−(aq); acidic solution
Step 1: Assign oxidation numbers to each species
Cl2 has a oxidation state of 0
Cl in ClO3− has an oxidation state of +5
Cl− has an oxidation state of -1
that means Cl2 went through reduction to form Cl− and went through oxidation to form ClO3−
Step 2: Determine the reduction/oxidation half-reaction
Reduction: Cl2(aq) →Cl−(aq)
Oxidation:Cl2(aq) →ClO3−(aq)
Step 3: Balance the half reactions
Reduction:[2e-+Cl2(aq) →2Cl−(aq)] x 5
Oxidation:Cl2(aq) + 6 H2O(aq) →2ClO3−(aq) + 12H++10e-
Step 4: Multiply the reduction half-reaction so that the charges cancel
Reduction: 10e-+5Cl2(aq) →10Cl−(aq)
Oxidation:Cl2(aq) + 6 H2O(aq) →2ClO3−(aq) + 12H++10e-
Step 5: Simply the overall reaction
6H2O(aq)+ 6Cl2(aq) →2ClO3−(aq) +10Cl−(aq) + 12H+
Overall reaction: 3H2O(aq)+ 3Cl2(aq) →ClO3−(aq) +5Cl−(aq) + 6H+
5. CO32−(aq) + N2H4(aq) → CO(g) + N2(g); basic solution
Step 1: Assign oxidation numbers to each species
C in CO32−(aq) has an oxidation state of +4 while C in CO(g) has an oxidation state of +2 which means it went through reduction.
N in N2H4(aq) has an oxidation state of -2 while N in N2(g) has an oxidaiton state of 0 which means it went through oxidation.
Step 2: Determine the reduction/oxidation half-reaction
Reduction: CO32−(aq) → CO(g)
Oxidation: N2H4(aq) →N2(g)
Step 3: Balance the half reactions. Cancel out the electrons to keep charge neutral.
Reduction: [CO32−(aq) + 4H+(aq) + 2e- → CO(g) + 2H2O(aq) ] X 2
Oxidation: N2H4(aq) →N2(g) + 4H+(aq) + 4e-
Step 4: Write the overall reaction and add OH- to neutralize the H+. Don't forget to cancel out the H2O(aq) for simplest equation.
Reduction: 2CO32−(aq) + 8H+ (aq)+ 4e- → 2CO(g) + 4H2O(aq)
Oxidation: N2H4(aq) →N2(g) + 4H+(aq) + 4e-
Overall reaction: 2CO32−(aq)+N2H4(aq)+4H+(aq) +4OH-(aq)→N2(g)+2CO(g) + 4H2O(aq) +4OH-(aq)
Overall reaction: 2CO32−(aq)+N2H4(aq)→N2(g)+2CO(g) +4OH-(aq)
Q20.5.5
State whether you agree or disagree with this statement and explain your answer: Electrochemical methods are especially useful in determining the reversibility or irreversibility of reactions that take place in a cell.
S20.5.5
Since the electrochemistry and gibbs energy are related we can use it to determine whether if a reaction is reversible or irreversible by seeing if the K is big or small or or if the delta G is negative or positive. If K is big that means the reaction favors the products and same goes with negative delta ge which means the reaction is very favorable and will go towards the product side. This will most likely be irreversible since it's very favorable to form products. However if the K is small, then that means the reaction doesn't favor the product and that it reversible. If delta G is positive then the reaction is unfavorable and will most likely be reversible since it's not particularly favoring the product.
Phase II:
From the two equations which relate E° cell and gibbs freen energy, we can see that the more positive E°cell is, the more negative gibbs free energy will be. Based on this idea and the fact that we know spontaneous reaction results in negative Gibbs Free energy, therefore if E°cell is negative then the reaction can be reverse to get a spontaneous reaction, and if it is positive then it can't be reverse since it is already spontaneous and therefore favors the forward reaction. Likewise, if Gibbs free energy is negative than the forward reaction is favor, and if is positive then the reverse reaction is favor.
Q24.6.1
Describe crystal field theory in terms of its
- assumptions regarding metal–ligand interactions.
- weaknesses and strengths compared with valence bond theory.
S24.6.1
1. The assumption of the crystal field theory regarding metal-ligand interactions is that the interactions are purely electrostatic. In other words, crystal field theory explains that the electrostatic force binds ligands to metals. Therefore, the negative charged ligands will bind to positive metal ions because of the electrostatic force. As a result we need to consider spatial occupation of electrons in the d-orbitals including the electrons on the ligand field. The ligand field in general is repulsive because electrons are negative and repel each other. Crystal field theory also describes the electrostatic repulsion between the electrons in the d-orbitals of metals and the lone pairs on the ligand. We can determine whether of a complex has high spin or low-spin based on the crystal field splitting energy difference between the 2 d-orbitals of the metal when the ligand is attached. If the splitting energy is big, the pairing energy will be small, so it will be a low spin complex. When the splitting energy is small, the pairing energy will be big so it will be a high spin complex.
2. The valence bond theory suggests that covalent bonds form due to the overlap of atomic orbitals. The weakness of this theory compared to the crystal field theory is that it doesn't explain why certain molecules such as BF3, which doesn't have enough electron pairs in the central atom to form the observed covalent bonds. The crystal field theory attempts at describing magnetic properties of transition metals but it doesn't really explain the bonding of transition metals.
Q14.7.9
Most enzymes have an optimal pH range; however, care must be taken when determining pH effects on enzyme activity. A decrease in activity could be due to the effects of changes in pH on groups at the catalytic center or to the effects on groups located elsewhere in the enzyme. Both examples are observed in chymotrypsin, a digestive enzyme that is a protease that hydrolyzes polypeptide chains. Explain how a change in pH could affect the catalytic activity due to (a) effects at the catalytic center and (b) effects elsewhere in the enzyme. (Hint: remember that enzymes are composed of functional amino acids.
Q14.7.9
A. Enzymes have specific optimal pH ranges that it operates at. Once it falls off of this range, they will no longer function optimally and may even denature. For the catalytic center, it is particularly sensitive to the pH because it's where the reactions occurs. Certain pH ranges may alter the binding sites of the enzyme, making it less efficient or not functional at all, such as preventing the substrate from binding to the binding site.
B. Effects elsewhere could change the conformation of the enzyme since the side chains might interact with the catalytic sites under different pH conditions. Changing of the conformation of the enzyme may impact the function. Since enzymes are made of different functional amino acids, the side chains of the amino acids may respond differently under different pH, such as the interactions between amino acids as it is been translate from the ribosome.
End of section
17.1: Balancing Oxidation-Reduction Reactions
Q17.1.1
If a 2.5 A current is run through a circuit for 35 minutes, how many coulombs of charge moved through the circuit?
Tutorial 17.1.1
First, we know that the total charge of the system (Q, in coulombs) can be determined by the equation Q= (I) x (T)
I = current in amperes (A), and T for time in seconds;
Since we are giving the current and time, we just have to find the coulomb of charge by plugging in what is giving into the equation.
Step 1. convert 35 minutes to seconds.
35 minutes X 60 seconds/ 1 minute = 2100 seconds
Step 2. Plug in 2.5A and 2100 seconds into the Q = I x T equation
Q= (2.5A) x (2100 Seconds) = 5250 Columbs (note that amperes is equivalent to 1C/sec so the seconds cancels out)
Make sure the answer is in the appropriate significant figures
Answer: 5.3 X 103 Columbs
S17.1.1
5.3 × 103 C
Q17.1.2
For the scenario in the previous question, how many electrons moved through the circuit?
Tutorial 17.1.2
For this problem we can use the following equation:

Ne = # of moles of electron
I = current (amps)
T= time (seconds)
F= Faraday's Constant (96,485 Coulombs/mole)
1. Given that we know the top part of the equation (current x time), we can simply divide it by the faraday's constant to get how many moles of electrons are going through the circuit.
Ne = (5.3 x 103 Coulombs)/ (96,485 Coulombs/mole) = .5493 moles of electron
2. To find the number of electron flowing through the circuit we can multiple the moles of electron by the avogadro's number
(.5493 moles of electron) x (6.022 x 1023 mol-1) = 3.31 x 1022 numbers of electron
OR
Another method is to simply divide the total charge by the charge of an electron to find out how many electrons are flowing through the circuit.
1. We already know that the total charge of the circuit is 5.3 x 103 Coulombs, by dividing the charge of an individual electron, we can calculate the total number of electron in the circuit.
(5.3 x 103 Coulombs) / (1.6 x 10-19 Coulombs)= 3.31 × 1022 Number of electrons
S17.1.2
If the total charge passing through the cell is equal to 5.3 × 103 C, then the number of electrons passing can be obtained by dividing the total charge by the charge per electron.
therefore; no. of electrons = (5.3 × 103 C)/1.6 × 10-19 C
=3.31 × 1022 number of electrons moved through the circuit.
Q17.1.3
For each of the following balanced half-reactions, determine whether an oxidation or reduction is occurring.
- Fe3++3e ⟶Fe
- Cr⟶Cr3++3e−
- MnO42−⟶MnO4-+e−
- Li++e-⟶Li
Tutorial 17.1.3
Remember that reduction refers to gaining of electrons while oxidation is losing electrons. To determine if the half reaction is reduction or oxidation, we have to look at the reactant and the product see if the reactants lost or gained electrons to form the product. Assign oxidation numbers to each species to see whether if the species gained or lost electron.
1.Fe3++3e- --> Fe
Fe3+ has an oxidation state of 3 while Fe has an oxidation state of 0. To get from 3 to 0 we need to add 3 electrons.
The Fe3+ is gaining 3 electrons which means this reaction is a reduction reaction.
2. Cr --> Cr3++3e-
Cr has an oxidation state of 0 and Cr3+ has an oxidation state of 3. To get from oxidation state 0 to 3, Cr would have to lose 3 electrons to form Cr3+.
This indicates that Cr goes through oxidation and loses 3 electron to get to Cr3+ which means this is a oxidation reaction.
3.Looking at the species, the overall charge of MnO42− decreased by 1 to form MnO4- which means an oxidation reaction occurred. To verify this we can check the oxidation numbers of each individual species to see which species loss an electron
Mn in MnO42− has a charge of +6 since 4 O = 4 (-2)= -8 but the overall charge of the molecule is 2- which means Mn has to be +6.
Mn in MnO4- has a charge of +7 since 4 O = 4(-2)= -8 but the overall charge of the molecule is 1- which means Mn has to be +7
Mn therefore became more positive which means it lost one electron to become more positive.
This indicates that MnO42−⟶MnO4-+e− is a oxidation reaction.
4. Li+1 has a oxidation state of +1 while the product Li has an oxidation state of 0.
This means Li+1 gained an electron to from Li which means this is a reduction reaction.
S17.1.3
(a) reduction; (b) oxidation; (c) oxidation; (d) reduction
Q17.1.4
For each of the following unbalanced half-reactions, determine whether an oxidation or reduction is occurring.
- Cl-⟶Cl2
- Mn2+⟶MnO2
- H2⟶H+
- NO3-⟶NO
Tutorial 17.1.4
In order to determine whether if each equation is an reduction or oxidation equation we must balance the equation first. To do so you must count up the number of atoms of each element there is on each side of the equation. Balance the number of atoms so that both sides have the same number of atoms per element. Remember to always balance the hydrogens and oxygens last. If you need to balance the oxygens you can add water (H2O). Add H+ to balance out the hydrogens. Lastly make sure the charge is the same on both sides and you can add H+ or e- to balance the charge so that both sides are equal. By examining if the reactant lost or gain electrons we can determine whether if the reaction is oxidation or reduction reaction.
1.Cl-⟶Cl2
Step 1 balance the Cl so that both sides have the same number of Cl
2Cl-⟶Cl2
Step 2. Balance the charge
Since 2Cl- has a charge of -2, we need to add 2e- on the Cl2 so that both sides have the same charge since Cl2 has a overall charge of 0
2Cl-⟶Cl2 + 2e-
Step 3. Determine whether if this is an oxidation or reduction reaction.
The oxidation state of 2Cl- is -2 and the oxidation state of Cl2 is 0. So 2Cl- lost 2 electrons to form Cl2, this is an oxidation reaction.
2.Mn2+⟶MnO2
Step 1: Since Mn2+ doesn't have oxygen, we would need to add H2O to balance out the oxygen in MnO2
Mn2++2H2O ⟶MnO2
Step 2: Next we want to balance the H
Mn2++2H2O ⟶MnO2+4H+
Step 3: Lastly balance the charges of both side of the equation so that they are equal.
Since the charge on the reactant is 2+ while the charge on the product is 4+, we need too add 2 e- to
Mn2++2H2O ⟶MnO2+4H++2e-
The overall charge is now 2+ for both sides
Step 4: Determine whether if it's an oxidation or reduction reaction.
Since the oxidation state of Mn2+ was +2 but Mn in MnO2 has an oxidation state of +4. Mn2+ lost 2 electrons to form MnO2, this is an oxidation reaction.
3.H2⟶H+
Step 1: Balance the H on both sides
H2⟶2H+
Step 2: Balance the charge on both sides
H2⟶2H++2e-
The charge of the reactant side is 0 and the charge on the product side is 2+ so we need to add 2 electrons to the right side so that both sides have a charge of 0
Step 3: Determine whether if the reaction is an oxidation or reduction reaction
Since H2 has an oxidation state 0 and H+ has an oxidation state of +1. So H2 lost 2 electrons to form 2H+ this is an oxidation reaction.
4.NO3-⟶NO
Step 1: Since the nitrogens are the same on both sides we can start balancing the oxygens by adding H2O.
NO3-⟶NO + 2H2O
Step 2: Balance the hydrogens on the reactant side so that it's equal to the product side.
NO3-+4H+⟶NO + 2H2O
Step 3: Next you want to balance the charge on both sides so that they are equal. We can balance the charge by adding electrons to the left side.
The reactant side has an overall charge of +3 while the product side has an overall charge of 0 so we can add 3e- so both sides have an overall charge of 0
NO3-+4H+ +3e-⟶NO + 2H2O
Step 4: Determine whether if this is an oxidation or reduction reaction.
The nitrogen in the reactant side has an oxidation state of +5 while the nitrogen in the product side has an oxidation state of +2. So the reactant gained 3 electrons to reach the oxidation state of the product which means this is a reduction reaction
S17.1.4
(a) oxidation (b) oxidation (c) oxidation; (d) reduction
Q17.1.5
Given the following pairs of balanced half-reactions, determine the balanced reaction for each pair of half-reactions in an acidic solution.
- Ca⟶Ca2++2e−,F2+2e−⟶2F−
- Li⟶Li++e−,Cl2+2e−⟶2Cl−
- Fe⟶Fe3++3e−,Br2+2e−⟶2Br−
- Ag⟶Ag++e−,MnO4−+4H++3e−⟶MnO2+2H2O
Tutorial 17.1.5
When we are given a pair of half-reactions, first we need to determine which half reaction is the oxidation half-reaction and which one is the reduction half-reaction. Next we want to balance each oxidation/reduction half-reactions separately using the same rules as before (Balance the species that are not H or O first and then balance the H and the O using H2O and H+. Lastly balance out the charges of the reaction so that the overall charge of the reactant and the product are equal). However since these equations are already balanced, we will only have to multiply the equation when necessary by their common factor so that the electrons cancels. When we add the two half-reactions together we want the electrons to cancel so we would want to multiple the half reactions by the most simple common factor. Make sure to cancel out the H2O and H+ so that the net overall reaction is the simplest equation. Lastly since we are under acidic solution we do not need to add OH- to neutralize the H+ by forming water so leave the H+ as is.
1. Ca⟶Ca2++2e−,F2+2e−⟶2F−
Step 1: Determine the oxidation/reduction half-reactions
Since Ca is losing electrons to form Ca2+ it is the oxidation half reaction
O: Ca⟶Ca2++2e−
The F2 in the next reaction has a oxidation state of 0 and gained 2 electrons to form 2F- so this is a reduction half-reaction.
R: F2+2e−⟶2F−
Step 2: Balance each half-reaction
O: Ca⟶Ca2++2e−
R: F2+2e−⟶2F−
Both of these reactions are balanced so the electrons will cancel
Step 3: Write the overall reaction
F2+Ca⟶2F−+Ca2+
2. Li⟶Li++e−,Cl2+2e−⟶2Cl−
Step 1: Determine the oxidation/reduction half-reactions
Since Li is losing electrons to form Li+ it is the oxidation half reaction
O: Li⟶Li++e−
The Cl2 in the next reaction has a oxidation state of 0 and gained 2 electrons to form 2Cl- so this is a reduction half-reaction.
R:Cl2+2e−⟶2Cl−
Step 2: Balance each half-reaction
O: (Li⟶Li++e-) x 2
R:Cl2+2e−⟶2Cl−
Since the reduction reaction has 2 electrons, we have to multiply the oxidation reaction by a factor of 2 as well so that the electrons can cancel.
O: 2Li⟶2Li++2e-
R:Cl2+2e−⟶2Cl−
Cancel out the electrons and add the two equations together to get the overall balanced equation
Step 3: Write the overall reaction
Cl2+2Li⟶2Li++2Cl−
3. Fe⟶Fe3++3e−,Br2+2e−⟶2Br−
Step 1: Determine the oxidation/reduction half-reactions
Since Fe is losing electrons to form Fe3+ it is the oxidation half reaction
O: Fe⟶Fe3++3e−
The Br2 in the next reaction has a oxidation state of 0 and gained 2 electrons to form 2Br- so this is a reduction half-reaction.
R:Br2+2e−⟶2Br−
Step 2: Balance each half-reaction
O: (Fe⟶Fe3++3e−) x 2
R:(Br2+2e−⟶2Br−) x 3
Since the reduction reaction has 2 electrons while the oxidation reaction has 3 electrons, we have to multiply the oxidation reaction by a factor of 2 and the reduction reaction by a factor of 3 to get a common factor. That way the electrons can cancel.
O: 2Fe⟶2Fe3++6e-
R:3Br2+6e−⟶6Br−
Cancel out the electrons and add the two equations together to get the overall balanced equation
Step 3: Write the overall reaction
3Br2+2Fe⟶2Fe3++6Br−
4. Ag⟶Ag++e−, MnO4−+4H++3e−⟶MnO2+2H2O
Step 1: Determine the oxidation/reduction half-reactions
Since Ag is losing electrons to form Ag+ it is the oxidation half reaction
O: Ag⟶Ag++e−
The Mn in the next reaction has a oxidation state of +7 and gained 3 electrons to form MnO2 so this is a reduction half-reaction.
R:MnO4−⟶MnO2
Step 2: Balance each half-reaction
O: Ag⟶Ag++e−
R:MnO4−⟶MnO2
Since oxidation equation is balanced we will ballance the reduction equation and then go back and adjust the electron numbers before we add the 2 half reactions together.
2a. The product side only has 2 oxygens so we need to add H2O to balance out the oxygens. Since there are 4 on the reactant side we will multiple the H2O by 2
R: : MnO4−⟶MnO2 +2H2O
2b. Now that the oxygens are balanced we need to balance the H on the reactant side. We added 4H+ because there are 4H on the product side.
R: : MnO4−+ 4H+⟶MnO2 +2H2O
2c. Next we will balance the charge so that both sides have equal charges. We added 3 electrons so that the overall charge of the reactant side is also 0
R: : MnO4−+ 4H++3e-⟶MnO2 +2H2O
2d. Now we will bring in the oxidation equation and balance out the electrons on both half-reactions by multiplying the oxidation reaction by 3
O: (Ag⟶Ag++e−) x 3
2e.Cancel out the electrons and add the two equations together to get the overall balanced equation
R: : MnO4−+ 4H++3e-⟶MnO2 +2H2O
O: 3Ag⟶3Ag++3e−
Step 3: Write the overall reaction
MnO4+4H++3Ag⟶3Ag++MnO2+2H2O
S17.1.5
- F2+Ca⟶2F−+Ca2+
- Cl2+2Li⟶2Li++2Cl−
- 3Br2+2Fe⟶2Fe3++6Br−;
- MnO4+4H++3Ag⟶3Ag++MnO2+2H2O
Q17.1.5B
Balance the following in acidic solution:
- H2O2+Sn2+⟶H2O+Sn4+
- PbO2+Hg⟶Hg22++Pb2+
- Al+Cr2O72−⟶Al3++Cr3+
Tutorial 17.1.5B
Same rules apply as 17.1.5 so refer back to section 17.1.5.
1. H2O2+Sn2+⟶H2O+Sn4+
Step 1: Determine the oxidation/reduction half-reactions
Since Sn2+ is losing electrons to form Sn4+ it is the oxidation half reaction
O: Sn2+⟶Sn4+
The other equation is a reduction half-reaction.
R:H2O2⟶H2O
Step 2: Balance each half-reaction
O: Sn2+⟶Sn4+
R:H2O2⟶H2O
Let's start with the oxidation half-reaction
2a. The product has a charge of +4 so we will add 2 electrons so that both sides have equal charge
O: Sn2+⟶Sn4++ 2e-
2b. Next we will balance the reduction half-reaction
R: H2O2⟶H2O
We want both sides to equal number of oxygen so we will mutliple the H2O by 2
R: H2O2⟶2H2O
Now that the oxygens are balanced, we need the H on the reactant side to balance. So we will add 2H+ to the reactant side.
R: H2O2+2H+⟶2H2O
Lastly we want the charge in the reduction half-reaction to balance so we will add 2e- to the reactant side since the product side has overall charge of 0.
R: H2O2+2H++2e-⟶2H2O
2c. Now we will bring in the oxidation equation and balance out the electrons on both half-reactions and cancel out the electrons. Then add the two equations together to get the overall balanced equation. Since we are in acidic solution we do not need to neutralize the H+
O: Sn2+⟶Sn4++ 2e-
R: H2O2+2H++2e-⟶2H2O
Step 3: Write the overall reaction
H2O2+2H++Sn2+⟶Sn4++2H2O
2. PbO2+Hg⟶Hg22++Pb2+
Step 1: Determine the oxidation/reduction half-reactions
The oxidation state of Hg is 0 and Hg22+is +2 so Hg lost electrons to form Hg22+ . Since Hg is losing electrons to form Hg22+ it is the oxidation half reaction
O: Hg⟶Hg22+
Pb in the reactant has a oxidation number of +4 since 2 oxygen has -4 charge but the overall species has a charge of 0. So PbO2 gained electrons to form Pb2+. This is a reduction half-reaction.
R:PbO2⟶Pb2+
Step 2: Balance each half-reaction
O: Hg⟶Hg22+
R:PbO2⟶Pb2+
Let's start with the oxidation half-reaction
2a. Since there are 2Hg in the product we will multiple the reactant by 2
O: 2Hg⟶Hg22+
2b.Then we can add electrons to the product side so the charge is 0 for both sides
O:2Hg⟶Hg22++2e-
2c. Next we will balance the reduction half-reaction
R: PbO2⟶Pb2+
We want both sides to equal number of oxygen so we will add 2 H2O
R: PbO2⟶Pb2++ 2 H2O
Now that the oxygens are balanced, we need the H on the reactant side to balance. So we will add 4H+ to the reactant side.
R: PbO2 + 4H+⟶Pb2+ + 2 H2O
Lastly we want the charge in the reduction half-reaction to balance so we will add 2e- to the reactant side since the product side has overall charge of 0.
R: PbO2 + 4H++2e-⟶Pb2++2H2O
2d. Now we will bring in the oxidation equation and balance out the electrons on both half-reactions and cancel out the electrons. Then add the two equations together to get the overall balanced equation. Since we are in acidic solution we do not need to neutralize the H+
O: 2Hg⟶Hg22++2e-
R: PbO2 + 4H++2e-⟶Pb2++2H2O
Step 3: Write the overall reaction
2Hg+PbO2 +4H+⟶Hg22++Pb2++2H2O
3. Al+Cr2O72−⟶Al3++Cr3+
Step 1: Determine the oxidation/reduction half-reactions
Since Al is losing electrons to form Al3+ it is the oxidation half reaction
O: Al⟶Al3+
The other equation is a reduction half-reaction because the Cr went from a oxidation state of +6 in the reactant to oxidation state of +3
R:Cr2O72−⟶Cr3+
Step 2: Balance each half-reaction
O: Sn2+⟶Sn4+
R:H2O2⟶H2O
Let's start with the oxidation half-reaction
2a. The product has a charge of +4 so we will add 2 electrons so that both sides have equal charge
O: Sn2+⟶Sn4++ 2e-
2b. Next we will balance the reduction half-reaction
R: H2O2⟶H2O
We want both sides to equal number of oxygen so we will multiple the H2O by 2
R: H2O2⟶2H2O
Now that the oxygens are balanced, we need the H on the reactant side to balance. So we will add 2H+ to the reactant side.
R: H2O2+2H+⟶2H2O
Lastly we want the charge in the reduction half-reaction to balance so we will add 2e- to the reactant side since the product side has overall charge of 0.
R: H2O2+2H++2e-⟶2H2O
2c. Now we will bring in the oxidation equation and balance out the electrons on both half-reactions and cancel out the electrons. Then add the two equations together to get the overall balanced equation. Since we are in acidic solution we do not need to neutralize the H+
O: Sn2+⟶Sn4++ 2e-
R: H2O2+2H++2e-⟶2H2O
Step 3: Write the overall reaction
H2O2+2H++Sn2+⟶Sn4++2H2O
Q17.1.6
Identify the species that undergoes oxidation, the species that undergoes reduction, the oxidizing agent, and the reducing agent in each of the reactions of the previous problem.
S17.1.6
- oxidized: (a) Sn2+; (b) Hg; (c) Al
- reduced: (a) H2O2; (b) PbO2; (c) Cr2O2−7Cr2O72−
- oxidizing agent: (a) H2O2; (b) PbO2; (c) Cr2O2−7Cr2O72−
- reducing agent: (a) Sn2+; (b) Hg; (c) Al
Q17.1.7
Balance the following in basic solution:
(a) SO3 2−(aq) + Cu(OH)2(s) ⟶ SO4 2−(aq) + Cu(OH)(s)
(b) O2(g) + Mn(OH)2(s) ⟶ MnO2(s)
(c) NO3 −(aq) + H2(g) ⟶ NO(g)
(d) Al(s) + CrO4 2−(aq) ⟶ Al(OH)3(s) + Cr(OH)4 −(aq)
Q17.1.8
Identify the species that was oxidized, the species that was reduced, the oxidizing agent, and the reducing agent in each of the reactions of the previous problem.
S17.1.8
Oxidized = reducing agent: (a) SO2−3SO32−; (b) Mn(OH)2; (c) H2; (d) Al; reduced = oxidizing agent: (a) Cu(OH)2; (b) O2; (c) NO−3NO3−; (d) CrO2−4CrO42−
Q17.1.9
- Why is it not possible for hydroxide ion (OH−) to appear in either of the half-reactions or the overall equation when balancing oxidation-reduction reactions in acidic solution?
- Why is it not possible for hydrogen ion (H+) to appear in either of the half-reactions or the overall equation when balancing oxidation-reduction reactions in basic solution?
S17.1.9
In basic solution, [OH−] > 1 × 10−7 M > [H+]. Hydrogen ion cannot appear as a reactant because its concentration is essentially zero. If it were produced, it would instantly react with the excess hydroxide ion to produce water. Thus, hydrogen ion should not appear as a reactant or product in basic solution.
Q17.1.10
Why must the charge balance in oxidation-reduction reactions?
S17.1.10
There is no net change in the number of electrons in an oxidation-reduction reaction because electrons given off the oxidation half reaction are taken up by another species in the reduction half reaction. The charge must balance in oxidation-reduction reactions because they consist of half reactions with a reduced half and an oxidized half that occur simultaneously.
17.2: Galvanic Cells
Q17.2.1
Write the following balanced reactions using cell notation. Use platinum as an inert electrode, if needed.
- Mg(s)+Ni2+(aq)⟶Mg2+(aq)+Ni(s)Mg(s)+Ni2+(aq)⟶Mg2+(aq)+Ni(s)
- 2Ag+(aq)+Cu(s)⟶Cu2+(aq)+2Ag(s)2Ag+(aq)+Cu(s)⟶Cu2+(aq)+2Ag(s)
- Mn(s)+Sn(NO3)2(aq)⟶Mn(NO3)2(aq)+Au(s)Mn(s)+Sn(NO3)2(aq)⟶Mn(NO3)2(aq)+Au(s)
- 3CuNO3(aq)+Au(NO3)3(aq)⟶3Cu(NO3)2(aq)+Au(s)3CuNO3(aq)+Au(NO3)3(aq)⟶3Cu(NO3)2(aq)+Au(s)
S17.2.1
(a) Mg(s)│Mg2+(aq)║Ni+(aq)│Ni(s)Mg(s)│Mg2+(aq)║Ni+(aq)│Ni(s); (b) Cu(s)│Cu2+(aq)║Ag+(aq)│Ag(s)Cu(s)│Cu2+(aq)║Ag+(aq)│Ag(s); (c) Mn(s)│Mn2+(aq)║Sn2+(aq)│Sn(s)Mn(s)│Mn2+(aq)║Sn2+(aq)│Sn(s); (d) Pt(s)│Cu+(aq),Cu2+(aq)║Au3+(aq)│Au(s)Pt(s)│Cu+(aq),Cu2+(aq)║Au3+(aq)│Au(s)
Q17.2.2
Given the following cell notations, determine the species oxidized, species reduced, and the oxidizing agent and reducing agent, without writing the balanced reactions.
- Mg(s)│Mg2+(aq)║Cu2+(aq)│Cu(s)Mg(s)│Mg2+(aq)║Cu2+(aq)│Cu(s)
- Ni(s)│Ni2+(aq)║Ag+(aq)│Ag(s)Ni(s)│Ni2+(aq)║Ag+(aq)│Ag(s)
S17.2.2
a) Mg2+(aq) = oxidized ; Cu2+(aq) = reduced ; Mg2+(aq)= reducing agent ; Cu2+(aq)= oxidizing agent
b) Ni2+(aq) = oxidized ; Ag+(aq) = reduced ; Ni2+(aq) = reducing agent ; Ag+(aq) = oxidizing agent
Q17.2.3
For the cell notations in the previous problem, write the corresponding balanced reactions.
S17.2.3
(a) Mg(s)+Cu2+(aq)⟶Mg2+(aq)+Cu(s)Mg(s)+Cu2+(aq)⟶Mg2+(aq)+Cu(s); (b) 2Ag+(aq)+Ni(s)⟶Ni2+(aq)+2Ag(s)2Ag+(aq)+Ni(s)⟶Ni2+(aq)+2Ag(s)
Q17.2.4
Balance the following reactions and write the reactions using cell notation. Ignore any inert electrodes, as they are never part of the half-reactions.
- Al(s)+Zr4+(aq)⟶Al3+(aq)+Zr(s)Al(s)+Zr4+(aq)⟶Al3+(aq)+Zr(s)
- Ag+(aq)+NO(g)⟶Ag(s)+NO−3(aq)(acidic solution)Ag+(aq)+NO(g)⟶Ag(s)+NO3−(aq)(acidic solution)
- SiO2−3(aq)+Mg(s)⟶Si(s)+Mg(OH)2(s)(basic solution)SiO32−(aq)+Mg(s)⟶Si(s)+Mg(OH)2(s)(basic solution)
- ClO−3(aq)+MnO2(s)⟶Cl−(aq)+MnO−4(aq)(basic solution)ClO3−(aq)+MnO2(s)⟶Cl−(aq)+MnO4−(aq)(basic solution)
Q17.2.5
Identify the species oxidized, species reduced, and the oxidizing agent and reducing agent for all the reactions in the previous problem.
S17.2.5
Species oxidized = reducing agent: (a) Al(s); (b) NO(g); (c) Mg(s); and (d) MnO2(s); Species reduced = oxidizing agent: (a) Zr4+(aq); (b) Ag+(aq); (c) SiO2−3(aq)SiO32−(aq); and (d) ClO−3(aq)ClO3−(aq)
Q17.2.6
From the information provided, use cell notation to describe the following systems:
- In one half-cell, a solution of Pt(NO3)2 forms Pt metal, while in the other half-cell, Cu metal goes into a Cu(NO3)2 solution with all solute concentrations 1 M.
- The cathode consists of a gold electrode in a 0.55 M Au(NO3)3 solution and the anode is a magnesium electrode in 0.75 M Mg(NO3)2 solution.
- One half-cell consists of a silver electrode in a 1 M AgNO3 solution, and in the other half-cell, a copper electrode in 1 M Cu(NO3)2 is oxidized.
Q17.2.7
Why is a salt bridge necessary in galvanic cells like the one below?

In this standard galvanic cell, the half-cells are separated; electrons can flow through an external wire and become available to do electrical work.
S17.2.7
The salt bridge is necessary to maintain electrical neutrality within the internal circuit, keeping the redox reaction neutral. Without the salt bridge, the circuit would be open (or broken) and no current could flow. With a salt bridge, each half-cell remains electrically neutral and current can flow through the circuit. The salt bridge is required to maintain the charge neutrality in the cell. Therefore in the absence of a salt bridge, the charge will continue to build up on both electrodes and cell will lose its charge neutrality. A salt bridge consists of an ionic compound in is aqueous solution form that moves across cells to maintain the overall neutrality of the cell.
Q17.2.8
Pending
Q17.2.9
- An active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
- An active (metal) electrode was found to lose mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
S17.2.9
Active electrodes participate in the oxidation-reduction reaction. Since metals form cations, the electrode would lose mass if metal atoms in the electrode were to oxidize and go into solution. Oxidation occurs at the anode.
Q17.2.10
The mass of three different metal electrodes, each from a different galvanic cell, were determined before and after the current generated by the oxidation-reduction reaction in each cell was allowed to flow for a few minutes. The first metal electrode, given the label A, was found to have increased in mass; the second metal electrode, given the label B, did not change in mass; and the third metal electrode, given the label C, was found to have lost mass. Make an educated guess as to which electrodes were active and which were inert electrodes, and which were anode(s) and which were the cathode(s).
17.3: Standard Reduction Potentials
Q17.3.1
For each reaction listed, determine its standard cell potential at 25 °C and whether the reaction is spontaneous at standard conditions.
- Mg(s)+Ni2+(aq)⟶Mg2+(aq)+Ni(s)Mg(s)+Ni2+(aq)⟶Mg2+(aq)+Ni(s)
- 2Ag+(aq)+Cu(s)⟶Cu2+(aq)+2Ag(s)2Ag+(aq)+Cu(s)⟶Cu2+(aq)+2Ag(s)
- Mn(s)+Sn(NO3)2(aq)⟶Mn(NO3)2(aq)+Sn(s)Mn(s)+Sn(NO3)2(aq)⟶Mn(NO3)2(aq)+Sn(s)
- 3Fe(NO3)2(aq)+Au(NO3)3(aq)⟶3Fe(NO3)3(aq)+Au(s)3Fe(NO3)2(aq)+Au(NO3)3(aq)⟶3Fe(NO3)3(aq)+Au(s)
S17.3.1
(a) +2.115 V (spontaneous); (b) +0.4626 V (spontaneous); (c) +1.0589 V (spontaneous); (d) +0.727 V (spontaneous)
Q17.3.2
For each reaction listed, determine its standard cell potential at 25 °C and whether the reaction is spontaneous at standard conditions.
- Mn(s)+Ni2+(aq)⟶Mn2+(aq)+Ni(s)Mn(s)+Ni2+(aq)⟶Mn2+(aq)+Ni(s)
- 3Cu2+(aq)+2Al(s)⟶2Al3+(aq)+2Cu(s)3Cu2+(aq)+2Al(s)⟶2Al3+(aq)+2Cu(s)
- Na(s)+LiNO3(aq)⟶NaNO3(aq)+Li(s)Na(s)+LiNO3(aq)⟶NaNO3(aq)+Li(s)
- Ca(NO3)2(aq)+Ba(s)⟶Ba(NO3)2(aq)+Ca(s)Ca(NO3)2(aq)+Ba(s)⟶Ba(NO3)2(aq)+Ca(s)
Q17.3.3
Determine the overall reaction and its standard cell potential at 25 °C for this reaction. Is the reaction spontaneous at standard conditions?
Cu(s)│Cu2+(aq)║Au3+(aq)│Au(s)Cu(s)│Cu2+(aq)║Au3+(aq)│Au(s)
S17.3.3
3Cu(s)+2Au3+(aq)⟶3Cu2+(aq)+2Au(s)3Cu(s)+2Au3+(aq)⟶3Cu2+(aq)+2Au(s); +1.16 V; spontaneous
Q17.3.4
Determine the overall reaction and its standard cell potential at 25 °C for the reaction involving the galvanic cell made from a half-cell consisting of a silver electrode in 1 M silver nitrate solution and a half-cell consisting of a zinc electrode in 1 M zinc nitrate. Is the reaction spontaneous at standard conditions?
Q17.3.5
Determine the overall reaction and its standard cell potential at 25 °C for the reaction involving the galvanic cell in which cadmium metal is oxidized to 1 M cadmium(II) ion and a half-cell consisting of an aluminum electrode in 1 M aluminum nitrate solution. Is the reaction spontaneous at standard conditions?
S17.3.5
3Cd(s)+2Al3+(aq)⟶3Cd2+(aq)+2Al(s)3Cd(s)+2Al3+(aq)⟶3Cd2+(aq)+2Al(s); −1.259 V; nonspontaneous
Q17.3.6
Determine the overall reaction and its standard cell potential at 25 °C for these reactions. Is the reaction spontaneous at standard conditions? Assume the standard reduction for Br2(l) is the same as for Br2(aq).
S17.3.6
Pt(s)│H2(g)│H+(aq)║Br2(aq)│Br−(aq)│Pt(s)Pt(s)│H2(g)│H+(aq)║Br2(aq)│Br−(aq)│Pt(s)
17.4: The Nernst Equation
Q17.4.1
For the standard cell potentials given here, determine the ΔG° for the cell in kJ.
- 0.000 V, n = 2
- +0.434 V, n = 2
- −2.439 V, n = 1
S17.4.1
(a) 0 kJ/mol; (b) −83.7 kJ/mol; (c) +235.3 kJ/mol
Q17.4.2
For the ΔG° values given here, determine the standard cell potential for the cell.
- 12 kJ/mol, n = 3
- −45 kJ/mol, n = 1
Q17.4.3
Determine the standard cell potential and the cell potential under the stated conditions for the electrochemical reactions described here. State whether each is spontaneous or nonspontaneous under each set of conditions at 298.15 K.
- Hg(l)+S2−(aq,0.10M)+2Ag+(aq,0.25M)⟶2Ag(s)+HgS(s)Hg(l)+S2−(aq,0.10M)+2Ag+(aq,0.25M)⟶2Ag(s)+HgS(s)
- The galvanic cell made from a half-cell consisting of an aluminum electrode in 0.015 M aluminum nitrate solution and a half-cell consisting of a nickel electrode in 0.25 M nickel(II) nitrate solution.
- The cell made of a half-cell in which 1.0 M aqueous bromine is oxidized to 0.11 M bromide ion and a half-cell in which aluminum ion at 0.023 M is reduced to aluminum metal. Assume the standard reduction potential for Br2(l) is the same as that of Br2(aq).
S17.4.3
(a) standard cell potential: 1.50 V, spontaneous; cell potential under stated conditions: 1.43 V, spontaneous; (b) standard cell potential: 1.405 V, spontaneous; cell potential under stated conditions: 1.423 V, spontaneous; (c) standard cell potential: −2.749 V, nonspontaneous; cell potential under stated conditions: −2.757 V, nonspontaneous
Q17.4.4
Determine ΔG and ΔG° for each of the reactions in the previous problem.
Q17.4.5
Use the data in Table P1 to determine the equilibrium constant for the following reactions. Assume 298.15 K if no temperature is given.
- AgCl(s)⇌Ag+(aq)+Cl−(aq)AgCl(s)⇌Ag+(aq)+Cl−(aq)
- CdS(s)⇌Cd2+(aq)+S2−(aq)at 377 KCdS(s)⇌Cd2+(aq)+S2−(aq)at 377 K
- Hg2+(aq)+4Br−(aq)⇌[HgBr4]2−(aq)Hg2+(aq)+4Br−(aq)⇌[HgBr4]2−(aq)
- H2O(l)⇌H+(aq)+OH−(aq)at 25 °CH2O(l)⇌H+(aq)+OH−(aq)at 25 °C
S17.4.5
(a) 1.7 × 10−10; (b) 2.6 × 10−21; (c) 8.9 × 1019; (d) 1.0 × 10−14
17.5: Batteries and Fuel Cells
Q17.5.1
What are the desirable qualities of an electric battery?
Q17.5.2
List some things that are typically considered when selecting a battery for a new application.
S17.5.2
Considerations include: cost of the materials used in the battery, toxicity of the various components (what constitutes proper disposal), should it be a primary or secondary battery, energy requirements (the “size” of the battery/how long should it last), will a particular battery leak when the new device is used according to directions, and its mass (the total mass of the new device).
Q17.5.3
Consider a battery made from one half-cell that consists of a copper electrode in 1 M CuSO4 solution and another half-cell that consists of a lead electrode in 1 M Pb(NO3)2Pb(NO3)2solution.
- What are the reactions at the anode, cathode, and the overall reaction?
- What is the standard cell potential for the battery?
- Most devices designed to use dry-cell batteries can operate between 1.0 and 1.5 V. Could this cell be used to make a battery that could replace a dry-cell battery? Why or why not.
- Suppose sulfuric acid is added to the half-cell with the lead electrode and some PbSO4(s)PbSO4(s) forms. Would the cell potential increase, decrease, or remain the same?
Q17.5.4
Consider a battery with the overall reaction:
Cu(s)+2Ag+(aq)⟶2Ag(s)+Cu2+(aq)Cu(s)+2Ag+(aq)⟶2Ag(s)+Cu2+(aq)
- What is the reaction at the anode and cathode?
- A battery is “dead” when it has no cell potential. What is the value of QQ when this battery is dead?
- If a particular dead battery was found to have [Cu2+]=0.11M[Cu2+]=0.11M, what was the concentration of silver ion?
S17.5.4
(a)
anode:
Cu(s)⟶Cu2+(aq)+2e−Cu(s)⟶Cu2+(aq)+2e−
with Eo(anode)=0.34VEo(anode)=0.34V
cathode:
2×(Ag+(aq)+e−⟶Ag(s))2×(Ag+(aq)+e−⟶Ag(s))
with E∘(cathode)=0.7996VE∘(cathode)=0.7996V
(b) 3.5×10153.5×1015
(c) 5.6×10−9M5.6×10−9M
Q17.5.5
An inventor proposes using a SHE (standard hydrogen electrode) in a new battery for smartphones that also removes toxic carbon monoxide from the air:
- Anode:
CO(g)+H2O(l)⟶CO2(g)+2H+(aq)+2e−CO(g)+H2O(l)⟶CO2(g)+2H+(aq)+2e−
with Eo(anode)=−0.53VEo(anode)=−0.53V - Cathode:
2H+(aq)+2e−⟶H2(g)2H+(aq)+2e−⟶H2(g)
with Eo(cathode)=0VEo(cathode)=0V - Overall reaction:
CO(g)+H2O(l)⟶CO2(g)+H2(g)CO(g)+H2O(l)⟶CO2(g)+H2(g)
with Eocell=+0.53VEcello=+0.53V
Would this make a good battery for smartphones? Why or why not?
Q17.5.6
Why do batteries go dead, but fuel cells do not?
S17.5.6
Batteries are self-contained and have a limited supply of reagents to expend before going dead. Alternatively, battery reaction byproducts accumulate and interfere with the reaction. Because a fuel cell is constantly resupplied with reactants and products are expelled, it can continue to function as long as reagents are supplied.
Q17.5.7
Explain what happens to battery voltage as a battery is used, in terms of the Nernst equation.
Q17.5.8
Using the information thus far in this chapter, explain why battery-powered electronics perform poorly in low temperatures.
S17.5.8
EcellEcell, as described in the Nernst equation, has a term that is directly proportional to temperature. At low temperatures, this term is decreased, resulting in a lower cell voltage provided by the battery to the device—the same effect as a battery running dead.
17.6: Corrosion
Q17.6.1
Which member of each pair of metals is more likely to corrode (oxidize)?
- Mg or Ca
- Au or Hg
- Fe or Zn
- Ag or Pt
Q17.6.2
Consider the following metals: Ag, Au, Mg, Ni, and Zn. Which of these metals could be used as a sacrificial anode in the cathodic protection of an underground steel storage tank? Steel is mostly iron, so use −0.447 V as the standard reduction potential for steel. Mg and Zn
Q17.6.2
Aluminum (E∘Al3+/Al=−2.07V)(EAl3+/Al∘=−2.07V) is more easily oxidized than iron (E∘Fe3+/Fe=−0.477V)(EFe3+/Fe∘=−0.477V), and yet when both are exposed to the environment, untreated aluminum has very good corrosion resistance while the corrosion resistance of untreated iron is poor. Explain this observation.
Q17.6.3
If a sample of iron and a sample of zinc come into contact, the zinc corrodes but the iron does not. If a sample of iron comes into contact with a sample of copper, the iron corrodes but the copper does not. Explain this phenomenon.
S17.6.3
Both examples involve cathodic protection. The (sacrificial) anode is the metal that corrodes (oxidizes or reacts). In the case of iron (−0.447 V) and zinc (−0.7618 V), zinc has a more negative standard reduction potential and so serves as the anode. In the case of iron and copper (0.34 V), iron has the smaller standard reduction potential and so corrodes (serves as the anode).
Q17.6.4
Suppose you have three different metals, A, B, and C. When metals A and B come into contact, B corrodes and A does not corrode. When metals A and C come into contact, A corrodes and C does not corrode. Based on this information, which metal corrodes and which metal does not corrode when B and C come into contact?
Q17.6.5
Why would a sacrificial anode made of lithium metal be a bad choice despite its E∘Li+/Li=−3.04VELi+/Li∘=−3.04V, which appears to be able to protect all the other metals listed in the standard reduction potential table?
S17.6.5
While the reduction potential of lithium would make it capable of protecting the other metals, this high potential is also indicative of how reactive lithium is; it would have a spontaneous reaction with most substances. This means that the lithium would react quickly with other substances, even those that would not oxidize the metal it is attempting to protect. Reactivity like this means the sacrificial anode would be depleted rapidly and need to be replaced frequently. (Optional additional reason: fire hazard in the presence of water.)
17.7: Electrolysis
Q17.7.1
Identify the reaction at the anode, reaction at the cathode, the overall reaction, and the approximate potential required for the electrolysis of the following molten salts. Assume standard states and that the standard reduction potentials in Table P1 are the same as those at each of the melting points. Assume the efficiency is 100%.
- CaCl2
- LiH
- AlCl3
- CrBr3
Q17.7.2
What mass of each product is produced in each of the electrolytic cells of the previous problem if a total charge of 3.33 × 105 C passes through each cell? Assume the voltage is sufficient to perform the reduction.
- massCa=69.1gmassCl2=122gmassCa=69.1gmassCl2=122g
- massLi=23.9gmassH2=3.48gmassLi=23.9gmassH2=3.48g
- massAl=31.0gmassCl2=122gmassAl=31.0gmassCl2=122g
- massCr=59.8gmassBr2=276gmassCr=59.8gmassBr2=276g
Q17.7.3
How long would it take to reduce 1 mole of each of the following ions using the current indicated? Assume the voltage is sufficient to perform the reduction.
- Al3+, 1.234 A
- Ca2+, 22.2 A
- Cr5+, 37.45 A
- Au3+, 3.57 A
Q17.7.4
A current of 2.345 A passes through the cell shown in Figure for 45 minutes. What is the volume of the hydrogen collected at room temperature if the pressure is exactly 1 atm? Assume the voltage is sufficient to perform the reduction. (Hint: Is hydrogen the only gas present above the water?)
S17.7.4
0.79 L
Q17.7.5
An irregularly shaped metal part made from a particular alloy was galvanized with zinc using a Zn(NO3)2 solution. When a current of 2.599 A was used, it took exactly 1 hour to deposit a 0.01123-mm layer of zinc on the part. What was the total surface area of the part? The density of zinc is 7.140 g/cm3. Assume the efficiency is 100%.
This question is referring to the process of electroplating.
step (1) calculate the moles of electrons passing through the cell.
no. electrons= total charge/ charge on electron
=(2.599 A × 60 min× 60 seconds) / 1.6× 10-19 C
=(5.85× 1022 number of electrons)/ 6.02× 1022
= 0.971 mol of electrons
step (2) write the balanced equation
Zn2+ +2e- →Zn which tells us the stoichiometry of the reaction.
therefore moles of Zn = 0.486 mol
mass of Zn = 0.486 mol × 65.39 g/mol
= 31.8 grams of Zinc
therefore volume of zinc = (31.8 g) / (7.14 g/cm3 )
= 4.45 cm3
therefore the surface area = (4.45 cm3 ) / 0.0001123 cm = 396 m2