Extra Credit 25
- Page ID
- 82732
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 17.3.4
Determine the overall reaction and its standard cell potential at 25 °C for the reaction involving the galvanic cell made from a half-cell consisting of a silver electrode in 1 M silver nitrate solution and a half-cell consisting of a zinc electrode in 1 M zinc nitrate. Is the reaction spontaneous at standard conditions?
A17.3.4
Ag+ + e---> Ag (s) E0=0.8V
Zn (s) --> Zn2+ + 2e- E0 = -0.76V
So Zn is a better reducing agent than Ag and is more easily to perform oxidation.
Therefore, since this is a galvanic cell silver nitrate is the cathode and zinc nitrate is the anode
Ag+ (aq) + Zn(s) --> Zn2+ (aq)+ Ag(s)
E0=E0cathode-E0anode=0.8V - (-0.76V) = 1.56V > 0V
so the reaction is spontaneous, because E of the cell is positive, meaning that delta G will be negative.

Q 19.1.23
Dilute sodium cyanide solution is slowly dripped into a slowly stirred silver nitrate solution. A white precipitate forms temporarily but dissolves as the addition of sodium cyanide continues. Use chemical equations to explain this observation. Silver cyanide is similar to silver chloride in its solubility.
A. 19.1.23
As cyanide ions is slowly added into the solution, this reaction: Ag+(aq)+CN-(aq) ⟶ AgCN(s) takes place first. We can ignore the sodium and nitrate because they are spectator ions and will form sodium nitrate with the precipitate. This means that they are not participating in the reaction.
However, as there's more cyanide ions appearing in the solution, either Ag+(aq)+2CN-(aq)⟶[Ag(CN)2]-(aq) or AgCN(s)+CN-(aq)⟶[Ag(CN)2]-(aq) can take place since more CN- ions push these reaction toward left and produce more [Ag(CN)2]-, which is soluble. As you add more cyanide a different reaction occurs to balance the products and reactants.
Q 12.4.16
Fluorine-18 is a radioactive isotope that decays by positron emission to form oxygen-18 with a half-life of 109.7 min. (A positron is a particle with the mass of an electron and a single unit of positive charge; the nuclear equation is 18F⟶18O+e- Physicians use 18F to study the brain by injecting a quantity of fluoro-substituted glucose into the blood of a patient. The glucose accumulates in the regions where the brain is active and needs nourishment.
- What is the rate constant for the decomposition of fluorine-18?
- If a sample of glucose containing radioactive fluorine-18 is injected into the blood, what percent of the radioactivity will remain after 5.59 h?
- How long does it take for 99.99% of the 18F to decay?
A 12.4.16
a. k = 0.693/t1/2 = 0.00632 min-1 t(1/2)=109.7 this is consistent with it being a first order rate reaction
b. fraction remaining = 0.5t/t1/2 = 0.55.59*60/109.7 =12.0% The equation you use is ln[A t]/[A knot]=-kt
c. k = -1/t ln(A/A0) (remember that the number you use for you concentration final is not the 99.99 over 100 but 0.01 over a hundred, 99.99 has been used up and you want to use the final concentration.
t = 1458 min, it takes 1458 minutes to decay.
Q 21.2.10
Which of the following nuclei lie within the band of stability?
- argon-40
- oxygen-16
- 122Ba
- 58Ni
- 205Tl
- 210Tl
- 226Ra
- magnesium-24
A 21.2.1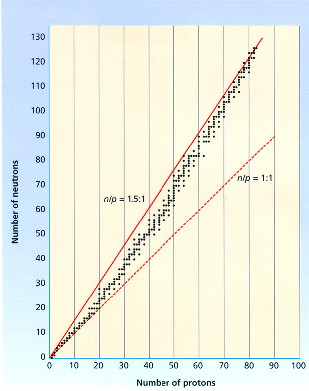
a,b,c,d,e,h lies within the band of stability.
Even numbers are more stable than odd numbers.
f: subtracting 210 with atomic number 81 for Ti, we will get a odd number--129, so its unstable.
The band of stability also stops at element 83 because there are no known stable isotopes above it.
g: Ra has 88 protons, which is larger than 83.
You can also calculate the number of neutrons by subtracting the mass number by the atomic number or the number of protons and look at a graph of the belt of stability in order to tell qualitatively.
Q 21.7.2
Based on what is known about Radon-222’s primary decay method, why is inhalation so dangerous?
A 21.7.2
Radon undergoes alpha decay. Alpha particles can be stopped by very thin shielding like skin or few cm of air, but have much stronger ionizing potential than beta particles, X-rays, and γ-rays. When inhaled, there is no protective skin covering the cells of the lungs, making it possible to damage the DNA in those cells and cause cancer. Alpha particles are fairly large, it is the nucleus of a helium atom, they can not get through a lot, that is why they are usually not dangerous on for us, but if they get inside they can do great damage due to their size, it can greatly cause DNA damage which could lead to a mutation and cancer.
Q 20.4.12
Draw the cell diagram for a galvanic cell with an SHE and a zinc electrode that carries out this overall reaction: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(g).
A 20.4.12
2H+(aq) + 2e- --> H2(g); E0 = 0.000 V cathode
Zn2+(aq) + 2e- --> Zn(s); E0= -076 V anode
Therefore, Zn is a better reducing agent than H.
Zn will perform oxidation and become the anode; H will perform reduction and become the cathode.
The electrode on the side of H2 needs to be inert, like Pt or graphite. They are good conductors but they themselves will not undergo redox chemistry.
Thus, the overall electrical cell looks like: Zn(s) | Zn2+ (aq) || H+ (aq) , H2(g) | Pt(s)

Q 20.4.14
A 20.4.14
Balancing the equation does not change the Ecell you do not need to multiply your reduction potential by the balancing number. They are independent of each other.
According to the reduction potential table.
E0=E0cathode-E0anode
a. Cu+(aq) + Ag+(aq) → Cu2+(aq) + Ag(s) E0=0.8V-0.16V=0.64V
b. Sn(s) + 2Fe3+(aq) → Sn2+(aq) + 2Fe2+(aq) E0=0.77V-(-0.14V) = 0.91V
c. Mg(s) + Br2(l) → 2Br−(aq) + Mg2+(aq) E0 = 1.09V-(-2.37V) = 3.46V
Q 14.1.3
What is the relationship between each of the following factors and the reaction rate: reactant concentration, temperature of the reaction, physical properties of the reactants, physical and chemical properties of the solvent, and the presence of a catalyst?
A 14.1.3
Reaction rates generally increase with increasing reactant concentration this is do to the fact that if you have more of the reactants then the concentration will push to the right wanting to balance it out, making it more spontaneous. Also if you think about it physically the more molecules that are there the more of a chance that they will collide which will increase the spontaneity of the reaction. Increasing temperature has the effect of increasing the speed of the molecules which also increases the probability that they will collide or react with each other. The addition of a catalyst lowers the activation energy which will increase the rate of the reaction; reaction rates decrease with decreasing reactant concentration, decreasing temperature, and removal of a catalyst.
Physical properties such as high solubility for reactants also increase reaction rates. Solvent polarity can either increase or decrease the reaction rate of a reaction, but increasing solvent viscosity generally decreases reaction rates.

