Extra Credit 20
- Page ID
- 82726
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)*Lauren's Phase II comments will be in blue
Q17.2.9
An active (metal) electrode was found to gain mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
Solution: The electrode was part of the cathode because the cathode underwent a reduction and the ion got reduced until it turns into a metal that binds to the active (metal) electrode. Cathodes attracts positively charges ions and cations since it is negatively charged electrodes. Since metals tend to be positively charged, the metal will bind to the negatively charged electrode and add mass to the electrode. In that case, the metal ions will flow from the anode to the cathode due to the positive and negative charges . Once the metal ions reach the cathode, it will bind to the cathode because opposite charged attractions. Therefore, the metal ions on the cathode resulted an increase in mass.
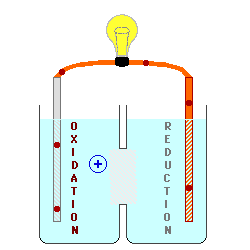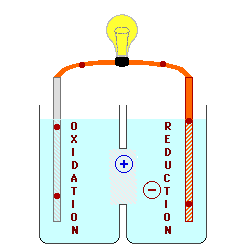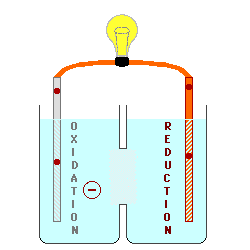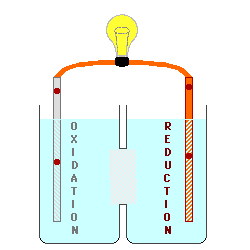
This image shows that ions are moving from the oxidation tank to the reduction tank. The oxidation tank signify anodes losing ions because the ions are attracted by the reduction tank (cathodes) and exemplifies why cathodes gain mass as the oxidation-reduction reaction is allowed to proceed.
I also agree that the cathode is involved because (thanks to the mnemonic OIL RIG) we know that the reduced material gains electrons and that the oxidized material loses electrons. When the reduced material (metal, in this case) gains electrons, it goes from the typically positively charged oxidation state to an oxidation state of 0, meaning it has gone from an aqueous ion to a solid metal. This solid metal has attracted to the cathode at which the reduction has taken place, explaining why it gained mass during the process.
- An active (metal) electrode was found to lose mass as the oxidation-reduction reaction was allowed to proceed. Was the electrode part of the anode or cathode? Explain.
Solution: The electrode was part of the anode because anodes go through oxidation and so there is a loss of electrons and makes anodes positively changed. When the oxidation-reduction reaction proceeds, metal ions repel themselves from the anode and flow towards the cathode side since the anode and the metal ions are both positively charged, they will repel themselves from each other and the metal ions will remove themselves from the electrode. This indicates a loss of mass as the oxidation-reduction reaction proceeds and shows why the electrode that loss mass was an anode. Anodes attract electrons and anions because the metal ions are positively charged, the anode will repel from the metal ions, which also means metal ions will remove themselves from the anode electrode, which also results a decrease in mass on the electrode.
I also agree that it was the anode that lost mass in this process. As mentioned above, oxidized materials lose electrons (that will go on to perform reduction) which forms a positively charged, aqueous metal ion that will detach from the electrode (the anode) and move to the solution. Because the anode loses these metal ions, this demonstrates the loss of mass that was mentioned.
Q19.1.18
Describe the electrolytic process for refining copper.
Solution: By electrolysis, copper can be refined and purely made. The reason why copper needs to remove the impurities is because it helps increase the electrical conductivity in electrical wire. You can refine copper and remove the impurities through electrolysis. Pure copper is important in making electrical wire, because it creates better electrical conductivity when transferring electricity. In order for better electrical conductivity, the impurities needs to be removed and this can be done by firing the impure copper to remove the impurities, such as sulfur, oxygen, etc. and shaping them into electrical anodes that can be used in electrolysis. Then the copper electrodes are placed into an electrical cell (into separate beakers) where electrical current can pass through the beakers and onto the electrodes. Through this process, the copper is stripped off of the anode and deposited onto the cathode. This process helps remove the impurities and refine copper because all the copper has been deposited onto the cathode all in one electrode. This process increases the weight of the cathode due to copper being deposited onto the cathode. This is a prime example of how to tell if an electrode is a cathode or an anode, as stated in Q17.2.9 above.
We want to refine copper because it may include various impurities (such as metals like gold, silver, and platinum), and removing these impurities helps increase the function of the copper as it is used in wires (as mentioned in detail above). The process for refining copper involves an electrolytic cell that contains two beakers. One contains an electrode (anode, specifically) made of the impure copper we want to refine, and the other contains an electrode (cathode, specifically) made of VERY pure copper. During this process, pure copper ions will move from the anode (impure copper) to the cathode (already pure copper), creating a larger mass of pure copper. The reason we are able to get pure copper ions only on the cathode is that it is more easily oxidized than its impurities (silver, gold, platinum) and those will stay behind on the anode.
Q19.3.10
Would you expect the complex [Co(en)_3]Cl_3 to have any unpaired electrons? Any isomers?
Solution: I would expect the complex \([Co(en)_3]Cl_{3}\) to not have any unpaired electrons because the ligand ethylenediamine has high energy and the metal cation \(Co\) has 6 d orbitals. Because this complex has a coordination number (number of bonds that the metal cation is bounded to) of 6 so it has a geometry of an octahedral. The high energy level from ethylenediamine indicates that there is a high splitting energy so the metal cation will have a low spin where the electron need to fill the bottom rows containing \(t_{2g}\) before filling the top row with electrons.
(weak) I− < Br− < S2− < SCN− < Cl− < NO3− < N3− < F− < OH− < C2O42− ≈ H2O <NCS− < CH3CN < NH3 < en < NO2− < CN− ≈ CO (strong)
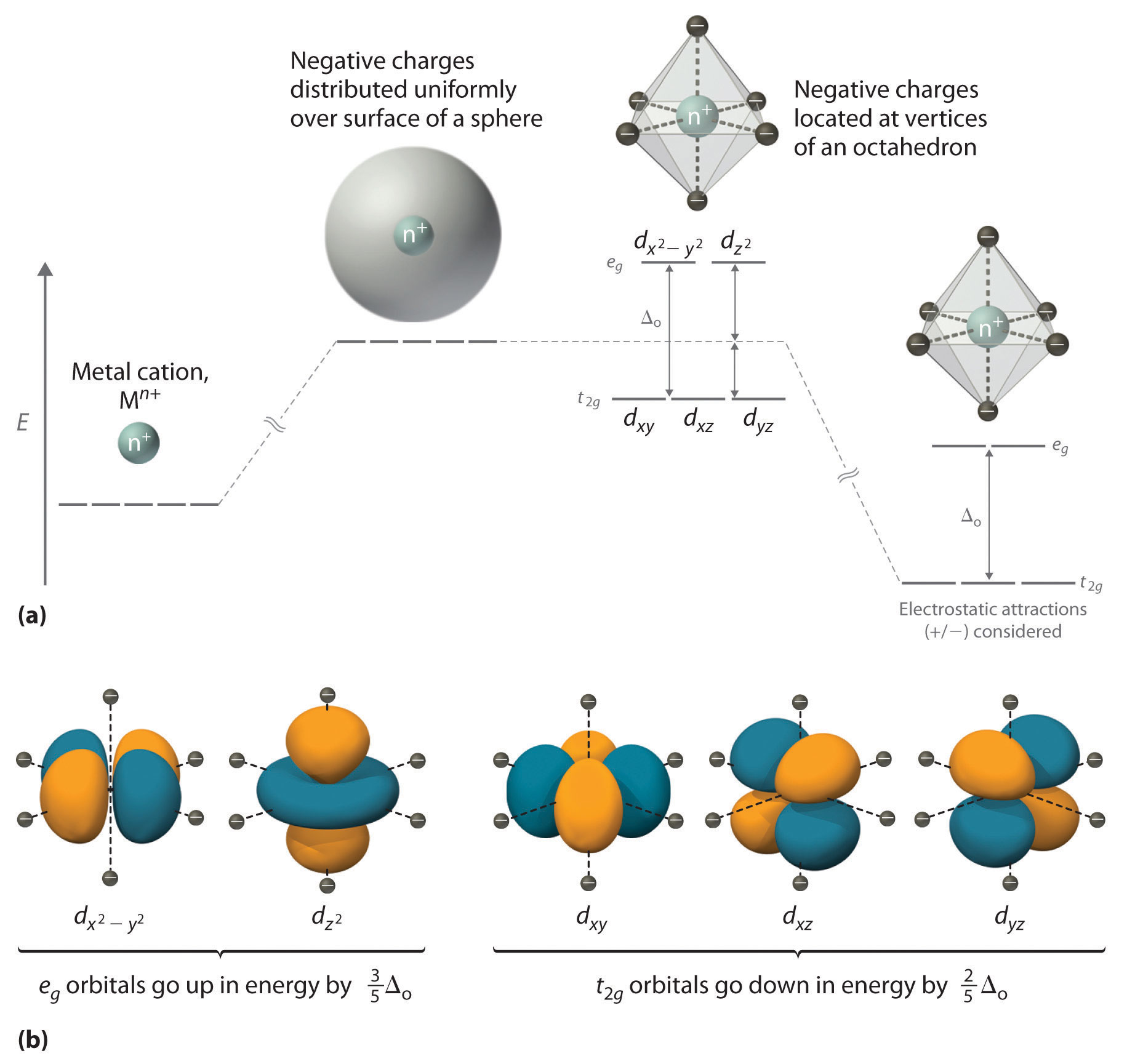
For more information about coordination numbers and crystal field spins, click on this link:
https://chem.libretexts.org/Core/Ino..._Field_Theory\
After doing the crystal field diagram, the diagram indicates no unpaired electrons and that the whole \(t_{2g}\) row has a pair of electron in each place. Because \([Co(en)_{3}]Cl_{3}\) does not have any geometric isomers and the mirrored image is not superimposable, it is an optical isomer. Optical isomers are complexes that are mirrored. Optical isomers are two compounds which contains the same number and kinds of atoms and bonds, while also having different arrangements of the atoms, but also contain non-superimposable mirror images. Since \([Co(en)_{3}]Cl_{3}\) doesn't have a superimposable mirrored image, we can conclude that it is an optical isomer
I agree, as (en) is a strong field ligand on the spectrochemical series, the crystal field splitting value will be larger. If this is larger, then a low spin formation will be favored over a high spin formation (it would rather pair electrons than overcome the crystal field splitting value). Also, because it has a coordination number of 6 (total number of attachments to the Co atom, with en attaching twice for each present, so 3x2=6 total), it will form an octahedral complex. The lowest energy levels will have 3 orbitals (t2g), each fitting two electrons. As (en) is neutral and 3 Cl- ions are needed to balance the complex ion, Co must have a +3 oxidation state. Co3+ has 6 electrons, so if the three bottom orbitals are completely filled before moving upwards to the eg orbitals, the 6 electrons will completely fill the t2g orbitals (3 orbitals with 2 electrons each equals 6), meaning there are no unpaired electrons. As far as isomers, this does not have any geometric isomers, however the mirror image of this coordination complex is NOT superimposable upon itself, so it has enantiomers (optical isomers) and is optically active. (For more information on how to identify optical isomers, look here: https://chem.libretexts.org/Core/Ino...anic_Complexes )
Q12.4.10
The reaction of compound A to give compounds C and D was found to be second-order in A. The rate constant for the reaction was determined to be 2.42 L/mol/s. If the initial concentration is 0.500 mol/L, what is the value of t1/2?
Solution: In order to find the value of t1/2 for second-order, we will need to know the equation for half life for second-order reactions. In class we learned that the rate law of a second order reaction is:
\[\dfrac{1}{[A]} = kt + \dfrac{1}{[A]_0} \label{6}\]
We will need to isolate t on it's own side so:
\[\dfrac{1}{[A]} -\dfrac{1}{[A]_0}= kt\]
where \(t=t_{1/2}\) and \([A]\) should be half of the initiation concentration so \({[A]}=\dfrac{[A]_0}{2}\) where
\[\dfrac{1}{[A]}=\dfrac{2}{[A]_0}\]
Then we get
\[\dfrac{2}{[A]_0} -\dfrac{1}{[A]_0}= kt_{1/2}\]
and when we isolate t on it's own to get the half life, we get the equation:
\[t_{1/2} = \dfrac{1}{k[A]_0} \label{7}\]
And now that we have the half life equation for second-order reactions, we plug in 2.42 L/mol/s for k and .500 mol/L for [A]_0 to get
\[t_{1/2} = \dfrac{1}{(2.42L/mol/s)(.500mol/L)}\]
When we plug everything in, we get the answer to be 0.826 and the moles and L cancel out so the units are seconds after proper cancelation.
\[t_{1/2}= 0.826 s \label{8}\]
The half-life equation that was derived above is correctly found as the same equation for second order reactions was also given in class. It is very important to make sure you are working with the correct equation as the rate equations and half-life equations will change depending on the order of the reaction (zero, first, second, etc). So using this correct equation and plugging the numbers in that were given in the instructions, we see that the units do correctly cancel out to give 0.826 seconds, which is the answer I got when I also calculated this.
Q21.2.5
Write the nuclide notation, including charge if applicable, for atoms with the following characteristics:
- 25 protons, 20 neutrons, 24 electrons
- 45 protons, 24 neutrons, 43 electrons
- 53 protons, 89 neutrons, 54 electrons
- 97 protons, 146 neutrons, 97 electrons
Solution:
When writing nuclide notation, the atomic number represents the number of protons in the atom. The atomic mass is the sum of protons and neutrons. When the number of electrons do not equal the number of protons, this indicates that the atom is charged. When there is a loss in electrons that means the atom is positively charged. When there is a gain in electrons, this indicates that the atom is negatively charged. Typically, the atomic mass is written on the top left corner and the atomic number is on the bottom left corner. The charges are placed on the top right corner of the atom.
\(atomic number=protons\) and \(atomic mass= protons+neutrons\) and the notation is written
\[ ^{X}_{Y}\text{Z}\] where \(X=atomic mass\) and \(Y=atomic number\) and \(Z=element symbol\)
1. Because there are 25 protons, that refers to the atomic number since it is stated that the number of protons equal the atomic number. So the molecule that has 25 protons refers to Manganese \(Mn\).
To find the atomic mass, you add the number of protons and neutrons together to get
\({25}+{20}={45}\) which represents the atomic mass of Mn
With the number of electrons, it indicates that there is a loss of an electron, which also means that the molecule is positively charged. So the overall nuclide notation would be:
\[ ^{45}_{25}\text{Mn} ^{+}\]
2. Using the same reasoning as above where the number of protons represent the atomic number, sum of protons and neutrons representing the atomic mass, and the electrons indicating the electrical charge of the atom, it tells me that the chemical element with an atomic number of 45 is Rhodium \(Rh\) and has a +2 charge because there is a loss in 2 electrons. When we add the number of protons and neutrons together, we get
\({45}+{24}={69}\), which represent the atomic mass of Rh.
The overall nuclide notation would be:
\[ ^{69}_{45}\text{Rh} ^{2+}\]
3. Using the same reasoning as above where the number of protons represent the atomic number, sum of protons and neutrons representing the atomic mass, and the electrons indicating the electrical charge of the atom, it tells me that the chemical element with an atomic number of 53 is Iodine(I) and has a +2 charge because there is a loss in 2 electrons. Combining the number of protons(53) and the number of neutrons(89), we get a sum of 142, which represent the atomic mass of the element.The overall nuclide notation would be:
\[ ^{142}_{53}\text{I} ^{-}\]
4. Using the same reasoning as above where the number of protons represent the atomic number, sum of protons and neutrons representing the atomic mass, and the electrons indicating the electrical charge of the atom, it tells me that the chemical element with an atomic number of 97 is Berkelium(Bk) and has a +0 charge because there there is no loss or gain of electrons. Combining the number of protons(97) and the number of neutrons(146), we get a sum of 243, which represent the atomic mass of the element. The overall nuclide notation would be:
\[ ^{243}_{97}\text{Bk}\]
In the beginning of the answers above, they mentioned the atomic number twice, first saying that it was the number of protons and electrons in the atom. The atomic number usually just denotes the number of protons, and this can be the same value as the number of electrons, but that is only true in neutrally charged atoms (as you will see in the results for the examples given above). The second time the atomic number was mentioned (right after the first definition), it was described as the sum of neutrons and protons in the atom. This would have been true if they had said "atomic mass" instead of "atomic number" again, but this was probably an unintentional mistake on their part. I can tell because later in the problem they showed a clear understanding of atomic mass, atomic number, and how the number of electrons plays a role in the charge of the atom (less electrons than protons means the atom is positively charged, and more electrons than protons means the atom is negatively charged). All of their nuclide notations matched mine exactly when I completed this problem.
After reading the commentary above, I have fixed my mistakes and made the corrections.
Q21.5.8
The mass of a hydrogen atom \(^{1}_{1}\text{H}\) is 1.007825 amu; that of a tritium atom \(^{3}_{1}\text{H}\) is 3.01605 amu; and that of an α particle is 4.00150 amu. How much energy in kilojoules per mole of \( ^{4}_{2}\text{He}\)
\( ^{4}_{2}\text{He}\) produced is released by the following fusion reaction: \(^{1}_{1}\text{H}\)+\(^{3}_{1}\text{H}\) \(\rightarrow\) \(^{4}_{2}\text{He}\)
Solution: In order to find the energy of He released by the fusion reaction, you will need to use the equation:
\[ E=mc^2 \]
where E is the energy released, m is the change in mass, and c as the speed of light.
Step 1) Find the sum of the mass of hydrogen and tritium atoms and subtract the sum from the mass of the alpha particle. This will give you the change in mass
\(^{1}_{1}\text{H}\)+\(^{3}_{1}\text{H}\)= 1.007825 amu+3.01605 amu= 4.023875amu
m=(\(^{1}_{1}\text{H}\)+\(^{3}_{1}\text{H}\))-\(^{4}_{2}\text{He}\)
\[m= 4.023875-4.00150=0.022375amu\]
This will give you the change in mass for the fusion reaction. However, this amount is in the units amu.
For E, the units have to be kJ per mol so we will convert amu to kg. We do this by multiplying the change in mass by 1.6605x10^-27.
0.022375amu x \(\dfrac{1.6605x10^-27kg}{1amu}\) = 3.715368x-29kg
This should yield to the answer: 3.715368x-29kg
Step 2) Then you would square the speed of light, which is a constant of 2.998x10^8 m/s and multiply that with the change in mass in kg that was found in step 1. This will yield the amount of energy that was released by the fusion reaction in J/ nucleus.
\[E=mc^2\]= \(E=(3.715368x-29kg)(2.998x10^8 m/s)^2\)=3.3393749x10^-12 J/nucleus.
Step 3) Next, you can convert Joules into kJ by dividing the number by 1000 joules to get kJ.
3.3393749x10^-12 J/nucleus x \(\dfrac{1kJ}{1000J}\)= 3.3393749x10^-15kJ/nucleus.
This would give us the answer: 3.3393749x10^-15kJ/nucleus.
Step 4) Finally, you can multiply the value by Avogadros number to get kJ/mol.
3.3393749x10^-15kJ/nucleus x \(\dfrac{6.022x10^23 nucleus}{1mole}\)=2010971577 kJ/mol
The final answer should be 2010971577 kJ/mol.
The process shown above is correct. In nuclear chemistry, mass can easily be converted to energy expended, so we use Einstein's equation E=mc2 to convert the mass lost (called the mass defect) into energy emitted. You would add the mass of the reactants (in amu) and subtract the mass of the products from this to find out how much of our original mass (that of the reactants) was lost. This value will be in amu, but you must convert it into kg in order to eventually arrive at joules and then kilojoules. This conversion factor is 1 amu = 1.6605x10-27kg. After finding this, we multiply the mass defect by the squared value of speed of light (2.998x108m/s). After squaring this and multiplying it by our mass defect, we end up with the units kg·m2/s2, which is the same unit as one Joule. After inputting the numbers and calculating these values, we end up with 3.339x10-12J/nucleus, but we want it in kilojoules, so simply divide this number by 1000 to get to kilojoules. This is still not the answer we are looking for, as it is in kJ/nucleus and not kJ/mole. To find this, multiply our value by Avogadro's number (6.022x1023), which has the units of nucleus/mole. After this, we arrive at the result 2010971576 kJ/mol or about 2.011x10-9kJ/mol. As you can see, my answer does match the one found above.
Q20.4.6
![]()
Explain why E° values are independent of the stoichiometric coefficients in the corresponding half-reaction.
Solution: E° values are independent in stoichiometric coefficients in the corresponding half-reactions because they are intensive properties where they do not depend on the amount of substance involved. The E° value depends on the standard conditions at
- A temperature of 298 K
- A pressure of gaseous components of 1 atm (or 1 bar)
- A concentration of 1 M
- Stoichiometry of electrons (not the substance)
so the coefficients of the substances involved has no affect of the E° value.
They are correct in that E° values do not depend on stoichiometric coefficients because standard reduction potentials are an intensive property. Intensive properties are those that do not depend on the amount of a material, but rather the specific material present (More on intensive properties here: https://chem.libretexts.org/Textbook...ive_Properties). The standard reduction potential just shows a constant value, so if the coefficients all increase by the same number, then the difference will not increase, it will still be the same (for an analogy, if I had 2 items in my left hand and 3 in my right hand, the ratio would be 2 to 3. If I doubled both, and had 4 and 6, the simplified ratio would still be 2 to 3).
Q20.7.4
![]()
Why are galvanic cells used as batteries and fuel cells? What is the difference between a battery and a fuel cell? What is the advantage to using highly concentrated or solid reactants in a battery?
Solution: Galvanic cells are used as batteries and fuel cells because they are rechargeable self-contained and portable. However, the major difference between batteries and the galvanic cells is that commercial batteries use solids or pastes rather than solutions as reactants to maximize the electrical output per unit mass. An obvious exception is the standard car battery which used solution phase chemistry. The difference between a battery and a fuel cell is that a battery runs down as the electrodes dissolve, but a fuel cell operates for as long as oxygen and hydrogen flow. In a battery, the electrodes are dissolved in a chemical reaction and in a fuel cell, no electrodes are consumed. Fuel cells can supply energy continuously, meanwhile batteries have a limited supply of energy (some batteries can be recharged also) This is what differentiates batteries from fuel cells.
These are all good points, and it is worth it to add to the first statement that not ALL batteries are rechargeable (primary batteries are not). It is also advantageous to use highly concentrated or solid reactants in batteries because it will last longer, and it will produce a steadier and more constant output of voltage than other less concentrated cells. For more information on galvanic cells and batteries, look here: https://chem.libretexts.org/Core/Ana...Galvanic_Cells

