Extra Credit 13
- Page ID
- 82718
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.2
Given the following cell notations, determine the species oxidized, species reduced, and the oxidizing agent and reducing agent, without writing the balanced reactions.
Answer 17.2.2
a. Mg(s)│Mg2+(aq)║Cu2+(aq)│Cu(s)
answer: -oxidation is when a species gives up electrons thus resulting in a more positively charged species therefore Mg(s) is oxidized
-reduction is when a species takes electrons thus resulting in a more negative charge therefore Cu2+(aq) is the species reduced
-the oxidizing agent is the species that is reduced thus Cu2+(aq) is the oxidizing agent
-the reducing agent is the species that is oxidized thus Mg(s) is the reducing agent
Phase II: No matter in a Galvanic cell (spontaneous) or a Voltaic cell (non spontaneous), oxidation will happen at the anode and reduction will happen in the cathode. In this cell notation, we see that the solid Mg loses two electrons and becomes Mg2+(aq) at the anode. At the cathode, Cu2+(aq) gains two more electrons and becomes Cu(s). Remember the acronym OIL RIG, oxidation is losing electrons and reduction is gaining electrons. Therefore, Mg(s) is oxidized and is a reducing agent and Cu2+ is reduced and is a oxidizing agent.
b. Ni(s)│Ni2+(aq)║Ag+(aq)│Ag(s)
answer: -oxidation is when a species gives up electrons thus resulting in a more positively charged species therefore Ni(s) is oxidized
-reduction is when a species takes electrons thus resulting in a more negative charge therefore Ag+(aq) is the species reduced
-the oxidizing agent is the species that is reduced thus Ag+(aq) is the oxidizing agent
-the reducing agent is the species that is oxidized thus Ni(s) is the reducing agent
Phase II: In this case, we see that Ni(s) loses two electrons to become Ni2+ (aq) and Ag+ gains an electron to became Ag(s). Therefore, Ni(s) is oxidized and Ag+ (aq) is reduced.
Q19.1.11
![]() Iron(II) can be oxidized to iron(III) by dichromate ion, which is reduced to chromium(III) in acid solution. A 2.5000-g sample of iron ore is dissolved and the iron converted into iron(II). Exactly 19.17 mL of 0.0100 M Na2Cr2O7 is required in the titration. What percentage of the ore sample was iron?
Iron(II) can be oxidized to iron(III) by dichromate ion, which is reduced to chromium(III) in acid solution. A 2.5000-g sample of iron ore is dissolved and the iron converted into iron(II). Exactly 19.17 mL of 0.0100 M Na2Cr2O7 is required in the titration. What percentage of the ore sample was iron?
Answer 19.1.11
answer: first we must determine how many moles Fe are produced for every mole Cr2O7. In order to do this we can balance the redox equation in acidic conditions.
first we will balance the reaction of Fe2+ to Fe3+
Fe2+ → Fe3+ + e-
Then we balance the reaction of Cr2O7 to Cr3+
Cr2O72- → 2Cr3+
Because Cr2O7 contains oxygen and Cr3+ does not, we will balance the equation by first adding H2O to the side with Cr3+
Cr2O72- → 2Cr3+ + 7H2O
now the left side needs to be balanced by adding the proper number of H+
Cr2O72- + 14H+ → 2Cr3+ + 7H2O
Finally we find that the reactants have a net charge of 12+ while the products have a net charge of 6+, therefore we must add 6 electrons to the left side to balance the charge
Cr2O72- + 14H+ + 6e- → 2Cr3+ + 7H2O
In order to combine the equations we have just determined they must have the same number of electrons, therefore, we multiply the iron redox equation by six
6Fe2+ → 6Fe3+ + 6e-
This gives us the total equation of
Cr2O72- + 14H+ + 6Fe2 → 2Cr3+ + 7H2O + 6Fe3+
Now we can begin to look at the titration part of this problem. We know it took 19.17 mL of NaCr2O7 to titrate and that the molarity of the NaCr2O7 was .01 M
From this we can use unit conversion to find the moles of NaCr2O7 in solution
19.17mL NaCr2O7 x (1L/103mL) x (.01 mol NaCr2O7/1L)= 1.917 x 10-4 mol NaCr2O7
1.917 x 10-4 mol NaCr2O7 = 1.917 x 10-4 mol Cr2O72-
From the equation we determined earlier we know that 1 mol Cr2O72- is equal to 6 mol Fe2+ therefore:
1.917 x 10-4 mol Cr2O72- x ( 6 mol Fe2+ / 1 mol Cr2O72-) = .0011502 mol Fe2+
Now, because we know the mol Fe2+ we can convert it to grams with the molar mass
0011502 mol Fe2+ x ( 55.85g Fe2+ / 1 mol Fe2+) = 0.06424 g Fe2+
0.06424 g Fe2+ = 0.06424 g Fe
Finally we will divide the amount of Fe we calculated by the total mass of the iron ore and mutilply by one hundred to find the percentage of the ore that was iron
(0.06424 g / 2.5g) x 100 = 2.57%
Q19.3.3
![]() Give the oxidation state of the metal, number of d electrons, and the number of unpaired electrons predicted for [Co(NH3)6]Cl3.
Give the oxidation state of the metal, number of d electrons, and the number of unpaired electrons predicted for [Co(NH3)6]Cl3.
Answer 19.3.3
Answer: In order to determine the oxidation state of Co we must look at the ligands, the outsphere ions, and the indicated overall charge of the complex. From this we see that the overall charge is zero. Therefore, the oxidation state of Co must balance out the negative charges on the other molecules and ions in the compound. When we look at the ligand we see that it is NH3 which does not have a charge. Then we look at the outsphere ions which consist of 3 chlorides. Because we know each chloride has a charge of -1 we can identify that in order for the compound to have a net charge of 0 Co must have a oxidation state of 3+
next we must identify the number of d electrons to do this we can first consider the electron configuration of Co without of charge
[Ar] 4s23d7
Therefore if Co took on a 3+ charge it would lose three of these electrons. We know once we get to the d orbital when removing electrons we remove electrons from the s orbital first and then the d orbital. Therefore, the electron configuration would become
[Ar]3d6
This shows that we have 6 d electrons
Finally we will determine the number of unpaired electrons. To do this we will need to look at whether NH3 is a strong or weak field electron; we will use the spectrochemical series in order to determine this
I− < Br− < S2− < SCN− < Cl− < NO3− < N3− < F− < OH− < C2O42− < H2O < NCS− < CH3CN < py < NH3
< en < bipy < phen < NO2− < PPh3 < CN− < CO
Looking at the spectrochemical series shows that NH3 is a strong field ligand and therefore would make the compound low spin. This means the the Octahederal splitting energy is greater than the pairing energy. Therefore all six electrons would pair in the lower t2g orbital leaving zero electrons unpaired
Phase II : According to the spectrochemical series of ligand, we see that NH3 is a strong field ligand. A strong field ligand means the compound has a very large octahedral splitting. Therefore, it is a low spin ligand. If we were to fill in the electrons in the way of high spin, then there will be five unpaired electrons. However, since NH3 is a low spin ligand, all six electrons will be filled in the t2g orbitals and it is a paramagnetic complex. Also, if we were to ask to determine the color of the complex, we could see that since the splitting is large, and there is a inverse relationship between splitting and wavelength, we will expect this compound to absorb a short wavelength and reflect color that is either be blue or violet (the color absorb will reflect it's complementary color).
Q12.4.3
Use the data provided in a graphical method to determine the order and rate constant of the following reaction:
2P⟶Q+W
| Time (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
|---|---|---|---|---|---|
| [P] (M) | 1.077 × 10−3 | 1.068 × 10−3 | 1.055 × 10−3 | 1.046 × 10−3 | 1.039 × 10−3 |
Answer 12.4.3
Answer: In order to determine the rate constant for this data we can graph the data with different axis. One graph will be of concentration vs. time, another of ln(concentration) vs. time and the final one of (1/concentration vs time). Once these graphs have been made we look for which one creates a straight line. the slope of that line is the rate constant.
using excel we find that this is the graph for concentration vs. time
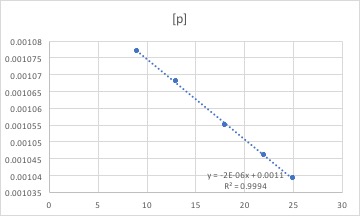
we find this is the graph for ln(concentration) vs. time

and we find this is the graph for (1/concentration) vs. time

Based on the data we have available to us in this problem we can not identify the order of this reaction. It is impossible for all the lines to be straight indicating that researcher has not collected enough data to allow us to determine the order. We can see this in how the concentrations in the table increase by very small amounts, thus only allowing us to look at a small portion of the overall reaction
Phase II: In order to determine the order of reaction, we could simply plot three different plots and see which one is linear. If concentration vs. time is linear, that means the rate increase disregard how the concentration will change, then the reaction is a zeroth order reaction. If ln(concentration) vs. time is linear, the reaction is a first order reaction and if 1/concentration vs. time is linear, the reaction is a second order reaction. However, in this question, just like the phase I person said, we cannot determine the rate of the concentration because all three plots are linear and it is hard to determine experimentally.
Q12.7.6
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
Answer 12.7.6
(a)
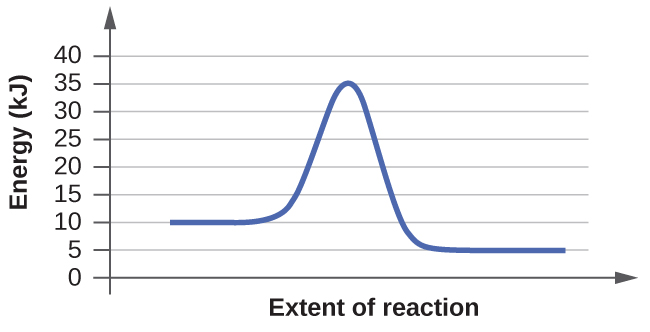
Answer for (a): In order to find activation energy based on the diagram all you must do is find the energy difference between the transition state and the reactants.
For this diagram:
35kj - 10kj =25kj
so the activation energy of the reaction is 25 kilojoules
(b)
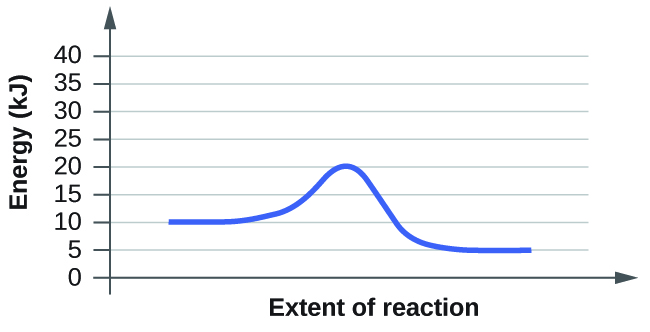
Answer for (b): In order to find activation energy based on the diagram all you must do is find the energy difference between the transition state and the reactants.
For this diagram:
20kj - 10kj =10kj
so the activation energy of the reaction is 10 kilojoules
Q21.5.1
Write the balanced nuclear equation for the production of the following transuranium elements:
- berkelium-244, made by the reaction of Am-241 and He-4
- fermium-254, made by the reaction of Pu-239 with a large number of neutrons
- lawrencium-257, made by the reaction of Cf-250 and B-11
- dubnium-260, made by the reaction of Cf-249 and N-15
Answer 21.5.1
Answers:
1. first we must set up the equation based on the given information. From the description we can tell that the reactants for this reaction are Am-241 and He-4, and product is berkelium-244. therefore:
\[\ce{^{241}_{95} Am} + \ce{^4_{2} He} → \ce{^{244}_{97} Bk}\]
Phase II: 
This reaction however is not balanced. On the right the mass numbers add to 245, but on the left we only have 244. The protons are balanced though as 95+2 (the atomic numbers of Am and He) sum to 97 which is the atomic number of Bk. Therefore to balance the equation we add a neutron to the product side because it will balance out the mass number difference. Therefore, our overall reaction is:
\[\ce{^{241}_{95} Am} + \ce{^4_{2}He} → \ce{^{244}_{97}Bk} + \ce{^{1}_{0} n}\]
Phase II: 
2. first we must set up the equation based on the given information. From the description we can tell that the reactants for this reaction are Pu-239 and a large number of neutrons, and the product is fermium-254. therefore:
\[\ce{^{239}_{94} Pu} + \ce{n} → \ce{^{254}_{100} Fm}\]
Phase II: 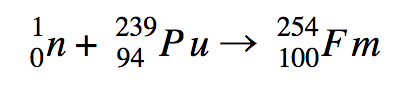
This reaction however is not balanced. On the right the mass number is 239 with an unspecified number of neutrons and on the left it is 254. The atomic numbers are also unbalanced; on the right we have 94 (atomic number of Pu) and on the left it 100 (atomic number of fermium).To fix this we can identify the number of neutrons in the reaction as 15. Therefore, on the right the mass numbers would total to 254 like on the right. to fix the imbalance on atomic numbers, on the left we can add 6 electrons so that the sum atomic number on the right would decrease 6 and equal 94. The overall reaction would be:
\[\ce{^{239}_{94} Pu} + \ce{15^{1}_{0} n} → \ce{^{254}_{100} Fm} + \ce{6^{0}_{-1} e}\]
Phase II: 
3. first we must set up the equation based on the given information. From the description we can tell that the reactants for this reaction are Cf-250 and B-11, and the product is lawrencium-257. Therefore:
\[\ce{^{250}_{98} Cf} + \ce{^{11}_{5} B} → \ce{^{257}_{103} Lr}\]
Phase II: 
The atomic numbers for this equation are balanced because 98 (atomic number Cf) + 11 (atomic number B) = 103 (atomic number Lr). The mass numbers for the reactants and products however are not balanced as the reactants sum to 261 and the product only has a mass number of 257. To fix this problem we can add 4 neutrons to the left side because each neutron will increase the mass number by 1 and not affect the atomic number. The overall reaction would be:
\[\ce{^{250}_{98} Cf} + \ce{^{11}_{5} B} → \ce{^{257}_{103} Lr} + \ce{4^{1}_{0} n}\]
Phase II: 
4. first we must set up the equation based on the given information. From the description we can tell that the reactants for this reaction are Cf-249 and N-15. The product is dubnium-260. therefore:
\[\ce{^{249}_{98} Cf} + \ce{^{15}_{7} N} → \ce{^{260}_{105} Db}\]
Phase II: 
The atomic numbers for this equation are balanced because 98 (atomic number Cf) + 7 (atomic number N) = 205 (atomic number Db). The mass numbers for the reactants and products however are not balanced as the reactants sum to 264 and the product only has a mass number of 260. To fix this problem we can add 4 neutrons to the left side because each neutron will increase the mass number by 1 and not affect the atomic number. The overall reaction would be:
\[\ce{^{249}_{98} Cf} + \ce{^{15}_{7} N} → \ce{^{260}_{105} Db} + \ce{4^{1}_{0} n}\]
Phase II: 
Q20.3.15
![]()
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
- 2Cl−(aq) + Br2(l) → Cl2(g) + 2Br−(aq)
- 2NO2(g) + 2OH−(aq) → NO2−(aq) + NO3−(aq) + H2O(l)
- 2H2O(l) + 2Cl−(aq) → H2(g) + Cl2(g) + 2OH−(aq)
- C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Answer 20.3.15
Answer (1)
The half reactions for this overall reaction are:
anode: 2Cl−(aq) → Cl2(g) + 2e−
cathode: Br2(l) + 2e− → 2Br−(aq)
We were able to determine the cathode versus the anode because oxidation (losing electrons) occurs at the anode and reduction (gaining electrons) occurs at the cathode.We find which side under goes oxidation versus reduction by determining oxidation numbers for the different species in the reaction. Species whose charge increase go through oxidation; species whose charge decreases go through reduction.
To determine whether or not the reaction will occur spontaneously will must determine if E°cell is positive or negative. A positive E°cell means that reaction will occur spontaneously and a negative E°cell means the reaction is not spontaneous. The equation to determine E°cell is E°cell = E°cathode - E°anode. To find the values of E°cathode and E°anode we use an SRP table. Thus:
anode: 2Cl−(aq) → Cl2(g) + 2e− E°=1.36
cathode: Br2(l) + 2e− → 2Br−(aq) E°=1.07
E°cell = 1.07 - 1.36
E°cell = -.29; therefore the reaction is non-spontaneous
Because the reaction is non-spontaneous in the given direction in order to write an cell diagram for a spontaneous reaction we must switch the anode and the cathode. When configuring cell diagrams the half reaction for oxidation goes on the left and the half reaction for reduction goes on the right. For species in different phases we will separate them using the symbol "|". Species in the same phase will have a comma between them. In between the two half reactions we will use the symbol "||" in order to represent the salt bridge. For half reactions that do not contain a solid we will use inert electrodes such a C(s) or Pt(s). The cell diagram for this reaction is:
Pt(s) | Br−(aq) | Br2(l) || Cl2(g) | Cl−(aq) | Pt(s)
Answer (2)
The half reactions for this overall reaction are:
anode: NO2(g) + e− → NO2−(aq)
cathode: NO2(g) + 2OH−(aq) → NO3−(aq) + H2O(l) + e−
We were able to determine the cathode versus the anode because oxidation (losing electrons) occurs at the anode and reduction (gaining electrons) occurs at the cathode. We find which side under goes oxidation versus reduction by determining oxidation numbers for the different species in the reaction. Species whose charge increase go through oxidation; species whose charge decreases go through reduction.
Next we must determine whether the reaction will occur spontaneously as written. For this problem details on the standard reduction potential for the oxidation reaction is not easily able to be found in most references so instead we will find the Gibbs energy of the equation to determine whether or not the cell is spontaneous.
To do this we must first find the individual Gibbs Energies for the species in the reaction
2NO2(g)51.3 + 2OH−(aq)-157.24 → NO2−(aq)-32.22 + NO3−(aq)-111.34 + H2O(l)-237.1
Then we will total the products and the reactants individual energies remembering to multiply by the coefficients
Products: [ (-32.22) + (-111.34) + (-237.1) ]
= -380.66 kj
reactants : [ 2(51.3) + 2(157.24) ]
= -211.888 kj
Next, we will subtract the total energy of our reactants from the total energy of our products
ΔG = (products) - (reactants)
=-380.66 - (-211.888)
= -168.772 kj
Because they reaction results in a negative ΔG we can confirm that this reaction is spontaneous
When configuring cell diagrams the half reaction for oxidation goes on the left and the half reaction for reduction goes on the right. For species in different phases we will separate them using the symbol "|". Species in the same phase will have a comma between them. In between the two half reactions we will use the symbol "||" in order to represent the salt bridge. For half reactions that do not contain a solid we will use inert electrodes such a C(s) or Pt(s). The cell diagram for this reaction is:
Pt(s) | NO2(g) | NO3−(aq) || NO2(g) | NO3−(aq) | Pt(s)
Answer (3)
The half reactions for this overall reaction are:
anode: 2Cl−(aq) → Cl2(g) + 2e−
cathode: 2H2O(l) + 2e− → H2(g) + 2OH−(aq)
We were able to determine the cathode versus the anode because oxidation (losing electrons) occurs at the anode and reduction (gaining electrons) occurs at the cathode. We find which side under goes oxidation versus reduction by determining oxidation numbers for the different species in the reaction. Species whose charge increase go through oxidation; species whose charge decreases go through reduction.
To determine whether or not the reaction will occur spontaneously will must determine if E°cell is positive or negative. A positive E°cell means that reaction will occur spontaneously and a negative E°cell means the reaction is not spontaneous. The equation to determine E°cell is E°cell = E°cathode - E°anode. To find the values of E°cathode and E°anode we use an SRP table. Thus:
anode: 2Cl−(aq) → Cl2(g) + 2e− E°=1.36
cathode: 2H2O(l) + 2e− → H2(g) + 2OH−(aq) E°cell = -0.83
E°cell = -0.83 - 1.36
E°cell = -2.19; therefore the reaction is non-spontaneous
Because the reaction is non-spontaneous in the given direction in order to write an cell diagram for a spontaneous reaction we must switch the anode and the cathode. When configuring cell diagrams the half reaction for oxidation goes on the left and the half reaction for reduction goes on the right. For species in different phases we will separate them using the symbol "|". Species in the same phase will have a comma between them. In between the two half reactions we will use the symbol "||" in order to represent the salt bridge. For half reactions that do not contain a solid we will use inert electrodes such a C(s) or Pt(s). The cell diagram for this reaction is:
Pt(s) | 2H2O(l) | H2(g) || Cl2(g) | Cl−(aq) | Pt(s)
Answer (4)
The half reactions for this overall reaction are:
anode: C3H8(g) + 6H2O(g) → 3CO2(g) + 20 H+(aq) + 20 e−
cathode: O2(g) + 4H+(aq) + 4 e− → 2H2O(g)
We were able to determine the cathode versus the anode because oxidation (losing electrons) occurs at the anode and reduction (gaining electrons) occurs at the cathode. We find which side under goes oxidation versus reduction by determining oxidation numbers for the different species in the reaction. Species whose charge increase go through oxidation; species whose charge decreases go through reduction.
Next we must determine whether the reaction will occur spontaneously as written. For this problem details on the standard reduction potential for the oxidation reaction is not easily able to be found in most references so instead we will find the Gibbs energy of the equation to determine whether or not the cell is spontaneous.
To do this we must first find the individual Gibbs Energies for the species in the reaction
C3H8(g)-23.4 + 5O2 (g)0 → 3CO2(g)-394.4 + 4H2O(l)-228.6
Then we will total the products and the reactants individual energies remembering to multiply by the coefficients
Products: [ 3(-394.4) + 4(-228.6) ]
= -2097.6 kj
reactants : [ (-23.4) + 5(0) ]
= -23.4 kj
Next, we will subtract the total energy of our reactants from the total energy of our products
ΔG = (products) - (reactants)
=-2097.6 - (-23.4)
= -2074.2 kj
Because they reaction results in a negative ΔG we can confirm that this reaction is spontaneous
When configuring cell diagrams the half reaction for oxidation goes on the left and the half reaction for reduction goes on the right. For species in different phases we will separate them using the symbol "|". Species in the same phase will have a comma between them. In between the two half reactions we will use the symbol "||" in order to represent the salt bridge. For half reactions that do not contain a solid we will use inert electrodes such a C(s) or Pt(s). The cell diagram for this reaction is:
Pt(s) | C3H8(g), CO2(g) || O2(g), H2O(g) | Pt(s)
Q20.5.28
Complexing agents can bind to metals and result in the net stabilization of the complexed species. What is the net thermodynamic stabilization energy that results from using CN−as a complexing agent for Mn3+/Mn2+?
Mn3+(aq) + e− → Mn2+(aq) E° = 1.51 V
Mn(CN)63−(aq) + e− → Mn(CN)64− E° = −0.24 V
Answer 20.5.28
Answer: This problem is asking us to find the total Gibbs energy for the reaction. A very negative Gibbs energy would mean that the complexed species is stable and favors the products while a positive Gibbs energy would indicate a reaction that is less stable and favors the reactants. Since we are given two E° values we will need to convert them individually to Gibbs energy values. We will flip the equation involving Mn(CN)64- so that the electrons will cancel out. This will make the E°= 0.24
The Nerst Equation is ΔG = -nFE° where n is the number of moles transferred and F is faraday's constant (96.485)
inputting our values we get:
ΔG = −(1)(96.485)(1.51)
= −146 kj/mole
and
ΔG = −(1)(96.485)(0.24)
=−23 kj
We can then sum these two values in order to find the net thermodynamic stabilization energy
−146 kj + −23kj = −169 kj/mole


