Extra Credit 12
- Page ID
- 82717
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)17.2.1:
Write the following balanced reactions using cell notation. Use platinum as an inert electrode, if needed.
a. \(\ce{Mg(s) + Ni^{2+}(aq)⟶Mg^{2+}(aq) + Ni(s)}\)
b. \(\ce{2Ag+(aq) + Cu(s)⟶Cu^{2+}(aq) + 2Ag(s)}\)
c. \(\ce{Mn(s) + Sn(NO3)2(aq)⟶Mn(NO3)2(aq) + Sn(s)}\)
d. \(\ce{3CuNO3(aq) +Au(NO3)3(aq)⟶3Cu(NO3)2(aq) +Au(s)}\)
Solution:
-
First divide cell reaction into oxidation and reduction half-reactions. Remember that oxidation occurs at the anode and reduction occurs at the cathode.
a. Oxidation: \(\ce{Mg(s) ->Mg^{2+}(aq) +2e-}\)
Reduction: \(\ce{Ni^{2+}(aq) +2e- ->Ni(s)}\)
b. Oxidation: \(\ce{Cu(s) -> Cu^{2+}(aq) + 2e-}\)
Reduction: \(\ce{Ag+(aq) +e- ->Ag(s)}\)
c. Oxidation: \(\ce{Mn(s) -> Mn^{2+}(aq) + 2e-}\)
Reduction: \(\ce{Sn^{2+}(aq) + 2e- -> Sn(s)}\)
d. Oxidation: \(\ce{Cu+(aq) +e- -> Cu^{2+}(aq)}\)
Reduction: \(\ce{Au^{3+}(aq) +3e- -> Au(s)}\) -
Write the anode/oxidation reaction on left side and cathode/reduction reaction on right side of cell notation, using one vertical line to indicate a phase change and two vertical lines to indicate the salt bridge between the anode and cathode. Electrons are not included in the cell notation, and phase is indicated in parentheses. An inert \(\ce{Pt(s)}\) electrode can be added to a side that does not have a solid electrode.
a. \(\ce{Mg(s) | Mg^{2+}(aq) || Ni+(aq) | Ni(s)}\)
b. \(\ce{Cu(s) | Cu^{2+}(aq) || Ag+(aq) | Ag(s)}\)
c. \(\ce{Mn(s) | Mn^{2+}(aq) || Sn^{2+}(aq) | Sn(s)}\)
d. \(\ce{Pt(s) | Cu+(aq), Cu^{2+}(aq) || Au^{3+}(aq) | Au(s)}\)
Note: An inert electrode is typically used when there is no phase change. It acts as a way to conduct the electricity when there is no solid to act as a physical electrode.
19.1.10:
Would you expect an aqueous manganese(VII) oxide solution to have a pH greater or less than 7.0? Justify your answer.
Solution:
- Lewis acid-base theory says that Lewis acids accept lone pair electrons or are electron pair acceptors. Bases donate lone pair electrons or are electron pair donors. Acids have a pH of less than 7.0 and bases have a pH of greater than 7.0.
-
Oxides can be acidic, basic, amphoteric or neutral in water. Typically they form bases when paired with a metal on the left side of the periodic table and acids when paired with a non-metal on the right side of the periodic table. Manganese is a transition metal on the right side of the periodic table.
-
Manganese(VII) Oxide, or \(\ce{Mn2O7}\) is known to oxidize readily, meaning that it loses electrons. This may indicate that it is a Lewis base and has a pH greater than 7.0.
-
However, in this case, Manganese(VII) Oxide, or \(\ce{Mn2O7}\) has one of the highest oxidation states for manganese, so manganese with an oxidation number of 7 will be able to accept many electrons, which means it is more acidic than manganese with lower oxidation numbers. Since manganese(VII) can accept so many electrons, it qualifies as a Lewis acid, and most probably has a pH of less than 7.0.
19.3.2:
Draw the crystal field diagrams for \(\ce{[Fe(NO2)6]^{4-}}\) and \(\ce{[FeF6]^{3-}}\). State whether each complex is high spin or low spin, paramagnetic or diamagnetic, and compare \(\ \Delta_{oct}\) to P for each complex.
Solution:
-
First, figure out how many electrons each compound has. \(\ce{[Fe(NO2)6]^{4-}}\) has 6 \(NO_2\) ligands so it has a coordination number of 6, which means it has 6 d electrons. Similarly, \(\ce{[FeF6]^{3-}}\) has 6 \(F^-1\) ligands so it also has coordination number of 6. However, the charge of the \(F^-\) ligand brings the charge down to -6, so Fe must have a charge of 3+ to compensate, which means it has 5 d electrons.
-
For both \(\ce{[Fe(NO2)6]^{4-}}\) and \(\ce{[FeF6]^{3-}}\), the geometry of the coordination complex is octahedral, meaning the crystal field splitting is the following:

-
Next, determine if the complex has a weak or strong field spin by looking at the spectrochemical series. If the complex is weak field, the splitting is lower, and the complex will be high-spin, while if the complex is strong field, it will be low-spin. Strong field and weak field are indicators of the \(∆_o\) energy. Strong field indicates that \(∆_o\) is large while low spin indicates that \(∆_o\) is low. When \(∆_o\) is low, then the energy required for electrons to pair is greater than the energy required to go to the next level (\(∆_o\) < P) whereas when \(∆_o\) is high, then the energy required for electrons to pair is lower than the energy required to go to the next level (\(∆_o\) > P). \(\ce{NO2-}\) is a strong field ligand, so it will induce a low spin complex, while \(\ce{F-}\) is a weaker field ligand, so it will induce a high-spin complex. If the complex is high spin, electrons will fill all the d-orbitals before pairing up, and low spin complexes fill the lowest energy orbitals first before moving to the higher energy orbitals.
-
Draw the crystal field diagram using the above image for the crystal field splitting.
-
Next, determine if the complex is diamagnetic or paramagnetic. If there are no unpaired electrons the complex is diamagnetic and if there are unpaired electrons the complex will be paramagnetic. Looking at the crystal field diagrams above, one can tell that \(\ce{[Fe(NO2)6]^{4-}}\) is diamagnetic while \(\ce{[FeF6]^{3-}}\) is paramagnetic.
-
If the complex is low-spin, \(\ P < \Delta _{oct}\), and if the complex is high-spin, \(\ P > \Delta _{oct}\).
12.4.2:
Use the data provided to graphically determine the order and rate constant of the following reaction: \(\ce{SO2Cl2 -> SO2+ Cl2}\)
|
Time (s) |
0 |
5.00 × 103 |
1.00 × 104 |
1.50 × 104 |
2.50 × 104 |
3.00 × 104 |
4.00 × 104 |
|---|---|---|---|---|---|---|---|
|
[SO2Cl2] (M) |
0.100 |
0.0896 |
0.0802 |
0.0719 |
0.0577 |
0.0517 |
0.0415 |
Solution:
-
Plot a graph of the data using \(\ce{[SO2Cl2]}\) vs. Time, ln\(\ce{[SO2Cl2]}\) vs. Time, and 1/\(\ce{[SO2Cl2]}\) vs. Time. These graphs are determined by the form of linearization for each of the integrated forms of the rate laws, where first order reactions are linearized with a logistic regression and second order reactions are linearized with an inverse regression.
-
In order to determine the order, look at the three graphs, and whichever one looks most linear is most likely the graph of the reaction. If \(\ce{[SO2Cl2]}\) vs. Time is linear, the reaction is zero order. If ln\(\ce{[SO2Cl2]}\) vs. Time is linear, the reaction is first order. If 1/ \(\ce{[SO2Cl2]}\) vs. Time is linear, the reaction is second order.


 The graph with the \(R^2\) value closest to one should be the order of the reaction. The graph of ln\(\ce{[SO2Cl2]}\) vs. Time has an \(R^2 = 1\), so the reaction is first order.
The graph with the \(R^2\) value closest to one should be the order of the reaction. The graph of ln\(\ce{[SO2Cl2]}\) vs. Time has an \(R^2 = 1\), so the reaction is first order. -
Since this is a first order reaction, the slope is the value of k determined by the graph of ln\(SO_2Cl_2\) vs Time. The value of the slope is \(-2\times 10^{-5}\), so k=\(-2\times 10^{-5}\) \(s^{-1}\).
12.7.5:
For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed:
(a)
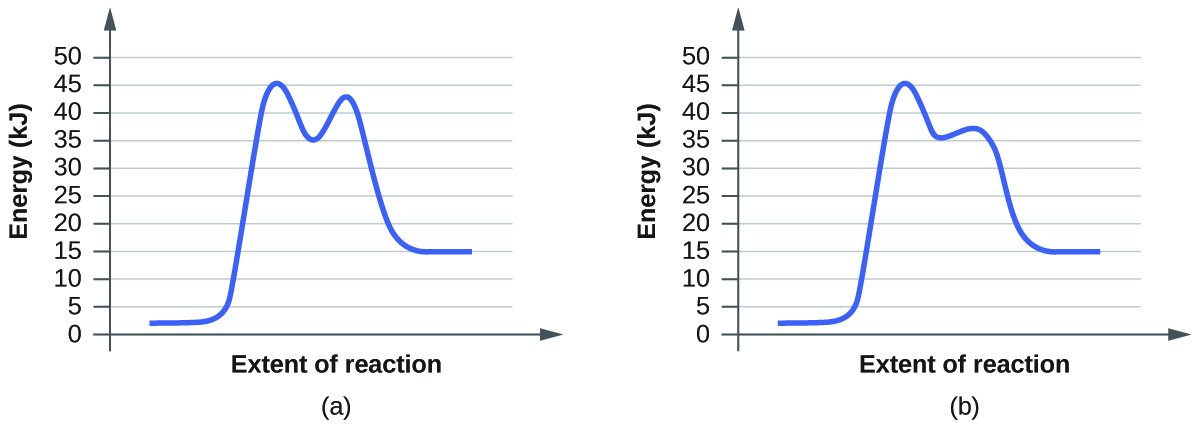
(b)
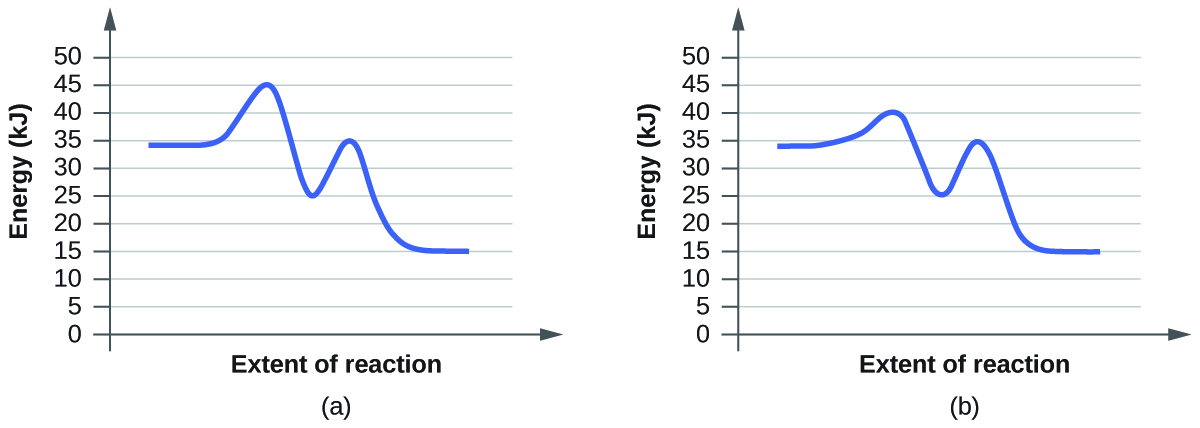
Solution:
A catalyzed reaction requires less activation energy than a non-catalyzed reaction. Therefore, in order to determine which reaction diagram shows the catalyzed reaction, you need to compare the activation energies of both diagrams. Keep in mind that the activation energy is the difference between the reactant and the transition states. The transition states are the highest points on the graph while the reactants are the very left of the graph.
a) The activation energies for (b) are lower than (a) since the difference between transition state energies are lower, so (a) is the catalyzed reaction.
b) The activation energies for (b) are lower than (a) since the difference between transition state energies are lower, so (b) is the catalyzed reaction.
21.4.28:
Write a balanced equation for each of the following nuclear reactions:
-
mercury-180 decays into platinum-176
-
zirconium-90 and an electron are produced by the decay of an unstable nucleus
-
thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay
-
neon-19 decays into fluorine-19
Solution:
In order to balance nuclear decay reactions, the mass number and atomic number must be balanced (equal) on both sides.
-
Determine the type of nuclear reaction that is occurring. In this case, mercury-180 decays into platinum-176, which involves a loss of 4 in the mass number and the loss of 2 protons, decreasing the atomic number by 2. Therefore, the reaction is likely alpha decay with an emission of an alpha particle, which is (\(\ce{^{4}_{2}He}\)). This is the case because both the mass number and atomic number have to be balanced on both sides of the equation.
\(\ce{^{180}_{80}Hg -> ^{4}_{2}He + ^{176}_{78}Pt}\) -
Determine the type of nuclear reaction that is occurring. Since an electron is produced, the reaction is a beta-minus decay, producing an (\_{-1}^{0}{e}\). \(^{90}_{40}Zr\) is also produced. That means that the original unstable nucleus is \(^{90}_{39}Y\). Therefore, the reaction is as follows: \[^{90}_{39}Y -> _{-1}^{0}{e} + ^{90}_{40}Zr\]
-
Determine the type of nuclear reaction that is occurring. There are 2 reactions here, an alpha decay and then a beta decay. The alpha decay produces \(\ce{^{4}_{2}He}\) while the beta decay produces \(\ce{^{0}_{-1}e}\).
\(\ce{^{232}_{90}Th -> ^{4}_{2}He + ^{228}_{88}Ra}\), \(\ce{^{228}_{88}Ra -> ^{0}_{-1}e + ^{228}_{89}Ac}\) -
Determine the type of nuclear reaction that is occurring. Since \(\ce{^{19}_{10}Ne}\) decays into \(\ce{^{19}_{9}F}\) the balanced equation must have a \(\ce{^{0}_{1}e}\) positron on the right side in order to balance the equation. This means the reaction that is occurring is \(\\beta^{+}\) decay.
\(\ce{^{19}_{10}Ne -> ^{0}_{1}e + ^{19}_{9}F}\)
To check your work, just add up the mass number and atomic numbers in the equation and see if they are equal on both sides of the reaction.
20.3.14:
For each redox reaction, write the half-reactions and draw the cell diagram for a galvanic cell in which the overall reaction occurs spontaneously. Identify each electrode as either positive or negative.
a. \(\ce{Ag(s) + Fe^{3+}(aq) -> Ag+(aq) + Fe^{2+}(aq)}\)
b. \(\ce{Fe^{3+}(aq) + 1/2H2(g) -> Fe^{2+}(aq) + H+(aq)}\)
Solution:
-
Split the reaction into two half-reactions. The oxidation half-reaction occurs at the anode while the reduction occurs at the cathode.
a. Anode: \(\ce{Ag(s) -> Ag+(aq) + e-}\) \(\text{E}^°_\text{cell} = 0.8 V\)
Cathode: \(\ce{Fe^{3+}(aq) + e- -> Fe^{2+}(aq)}\) \(\text{E}^°_\text{cell} = 0.77 V\)
b. Anode: \(\ce{1/2H2(g) -> H+(aq) + e-}\) \(\text{E}^°_\text{cell} = 0 V\)
Cathode: \(\ce{Fe^{3+}(aq) +e- -> Fe^{2+}(aq)}\) \(\text{E}^°_\text{cell} = 0.77 V\) -
Calculate spontaneity by standard reduction potentials using the equation \(\text{E}^°_\text{cell} = \text{E}^°_\text{cathode} - \text{E}^°_\text{anode}\). If \(\text{E}^°_\text{cell}\) is positive, then the reaction is spontaneous, if negative the reaction is not spontaneous.
a. \(\text{E}^°_\text{cell} = 0.77 V -0.8 V = -0.03 V\) , Negative and Not Spontaneous
b. \(\text{E}^°_\text{cell} = 0.77 - 0 V = 0.77 V\), Positive and Spontaneous -
For non-spontaneous reactions, reverse the reaction so that it is spontaneous. Write the anode/oxidation reaction on left side and cathode/reduction reaction on right side of cell notation, using one vertical line to indicate a phase change and two vertical lines to indicate the salt bridge between the anode and cathode. Electrons are not included in the cell notation, and phase is indicated in parentheses. An inert \(\ce{Pt(s)}\) electrode can be added to a side that does not have a solid electrode. If there is no solid phase, an inert electrode will be necessary.
a. \(Pt_{(s)} | Fe^{2+}_{(aq)}, Fe^{3+}_{(aq)} || Ag^{+}_{(aq)} | Ag_{(s)}\)
b. \(\ce{Pt(s) | H2(g) | H+(aq) || Fe^{3+}(aq) | Fe^{2+}(aq) | Pt(s)}\) -
Electrons from the oxidation half-reaction are released at the anode so the anode in a galvanic cell is negatively charged. The cathode, which attracts electrons, is positively charged.
20.5.27:
Under acidic conditions, ideally any half-reaction with E° > 1.23 V will oxidize water via the reaction \(\ce{O2(g) + 4H+(aq) + 4e- -> 2H2O(l)}\).
a. Will aqueous acidic \(\ce{KMnO4}\) evolve oxygen with the formation of \(\ce{MnO2}\)?
b. At pH 14.00, what is E° for the oxidation of water by aqueous \(\ce{KMnO4}\) (1 M) with the formation of \(\ce{MnO2}\)?
c. At pH 14.00, will water be oxidized if you are trying to form \(\ce{MnO2}\) from \(\ce{MnO4^{2-}}\) via the reaction \(2MnO_4^{2-} +2H_2O_{(l)} ⟶ 2MnO_{2_{(s)}} + O_{2_{(g)}} +4OH^-_{(aq)}\)?
Solution:
a. First determine the half-reactions needed to create the final reaction of \(\ce{MnO4–(aq) + 4H+ + 3 e– ->MnO2(s) + 2H2O(aq)}\).
\(\ce{MnO4-(aq) + 8 H+(aq) + 5 e- ->Mn2+(aq) + 4 H2O}\) \(\text{E}^°_\text{cell} = 1.51 V\)
\(\ce{MnO2(s) + 4 H+(aq) + 2 e- ->Mn2+(aq) + 2 H2O}\) \(\text{E}^°_\text{cell} = 1.23 V\)
Get \(\ce{E°}\) values from standard reduction tables.
We need to reverse the second reaction and add the 2 reactions in order to get the final reaction. However, you cannot just add the \(\ce{E°}\), you have to convert it to \(\Delta G\) using the equation \(\Delta G=-nFE\), where n is the number of moles of electrons and F is Faraday's Constant \(96485 C mol^{-1}\). After adding the \(\Delta G\), convert back to \(\ce{E°}\) using the same equation. In this case, n for the first reaction is 5, n for the second reaction is 2, and n for the overall reaction is 3.
\(\Delta G = -(5)(F)(1.51)\)
\(\Delta G = -(2)(F)(-1.23)\)
Sum: \((-5)(F)(1.51) -(2)(F)(-1.23) = -5.09F\)
\(E^{°}= -\frac{\Delta G}{nF} = -\frac{-5.09F}{3F} = 1.69 V\)
Since \(1.69 > 1.23\) this reaction will evolve oxygen. \(E^{°} = 0.46 V\)
b. First determine the half reactions needed to create the final reaction of \(\ce{MnO4-(aq) -> MnO2(s) + e- + O2(g)}\)
\(\ce{MnO4-(aq) + 2 H2O + 3 e- ->MnO2(s) + 4 OH-(aq)}\) \[ \text{E}^°_\text{cell} = 0.595 V\]
\(\ce{O2(g) + 2 H2O + 4 e- ->4 OH-(aq)}\) \[ \text{E}^°_\text{cell} = 0.4 V\]
Get \(\ce{E°}\) values from standard reduction tables. Keep in mind that for a basic reaction, a standard potential for \(\ce{O2}\) that has \(\ce{OH-}\) and not \(\ce{H+}\) should be used.
In this case, you can just subtract the reduction potential of the anode from the cathode in order to get the \(E^{°} = 0.595 - 0.4=0.195 V\)
c. First determine the half-reactions needed to create the final reaction of \(2MnO_4^{2-} +2H_2O_{(l)} ⟶ 2MnO_{2_{(s)}} + O_{2_{(g)}} +4OH^-_{(aq)}\).
\(\ce{2MnO4^{2-}(aq) + 4H2O + 4e- -> 2MnO2(s) + 8OH-(aq)}\) \[ \text{E}^°_\text{cell} = 0.6 V\]
\(\ce{O2(g) + 2 H2O + 4 e- ->4 OH-(aq)}\) \[ \text{E}^°_\text{cell} = 0.4 V\]
Get \(\ce{E°}\) values from standard reduction tables. Keep in mind that for a basic reaction, a standard potential for \(\ce{O2}\) that has \(\ce{OH-}\) and not \(\ce{H+}\) should be used.
The second reaction must be flipped and the 2 reactions added in order to get the final reaction. However, you cannot just add the \(\ce{E°}\), you have to convert it to \(\Delta G\) using the equation \(\Delta G=-nFE\), where n is the number of moles of electrons and F is Faraday's Constant \(96485 C mol^{-1}\). After adding the \(\Delta G\), convert back to \(\ce{E°}\) using the same equation. In this case, n for the first reaction is 2, n for the second reaction is 4, and n for the overall reaction is 2.
\(\Delta G = -(2)(F)(0.6)\)
\(\Delta G = -(4)(F)(-0.4)\)
Sum: \((-2)(F)(0.6) -(4)(F)(-0.4) = 0.4F\)
\(E^{°}= -\frac{\Delta G}{nF} = -\frac{0.4F}{2F} = 0.2 V\)
Since the answer is greater than zero, the reaction will oxidize oxygen. \(E^{°} = 0.20 V\)


