Extra Credit 11
- Page ID
- 82716
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Phase 1 student: Stephanie Tsai
Answers to assigned questions:
Section 12
Q 12.4.1
Describe how graphical methods can be used to determine the order of a reaction and its rate constant from a series of data that includes the concentration of A at varying times.
Answer:
The graphing method is especially useful when trying to determine an order of reaction when laboratory data is given.
The order of a reaction and rate constant can be easily determined by first first graphing the concentration of [A] and the correlating time in the following ways:
*Concentration is on the y-axis and time on the x-axis
- Graph (1) is [A] vs time-- indicative of zero order
- Graph (2) is Ln([A] vs time -- indicative of 1st order
- Graph (3) is 1/[A] vs time -- indicative of 2nd order
Once everything has been graphed, the graph with the most linear slope (you can make a straight line that touches every point on the drawn line) indicates the order of reaction. The rate constant can then be easily determined from the slope (rise/run) of the graph. An example of these graphs is provided below.

Explanation:
The graphing approach works because not only does each rate order have a unique effect on the reaction rate but also this particular question is only dealing with one reactant. Zero order reactions mean that the concentration of reactants do not affect the rate at which the reaction occurs. As a result, if the reaction is zero order, the series of data forms a straight line with a constant slope if the [A] and reaction time are graphed.
First order reactions change logarithmically, which is why the concentration of [A] must be natural "logged" to linearize it. If the data does not form a straight line, then the reaction is not first order. To clarify why this works, the rate of a first order reaction is -d[A]/dt = k[A] which when integrated can form a straight line of ln[A] = ln[A]0 - kt.
Second order reactions have an inverse concentration because its rate is represented by -d[A]/dt = k[A]2 which when integrated becomes linear with 1/[A] = 1/[A]0 +kt. If the data does not form a straight line, then the reaction is not second order.
Q 12.7.4
For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed:
Pair 1)
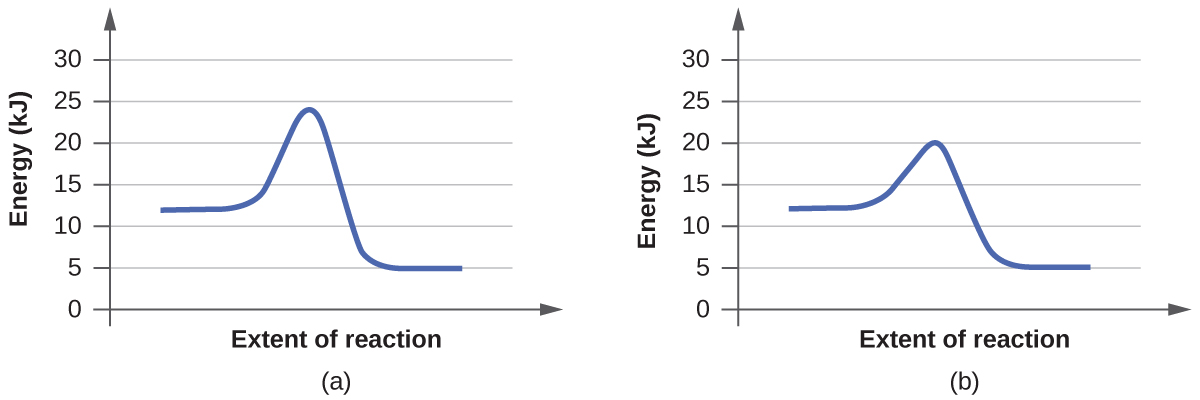
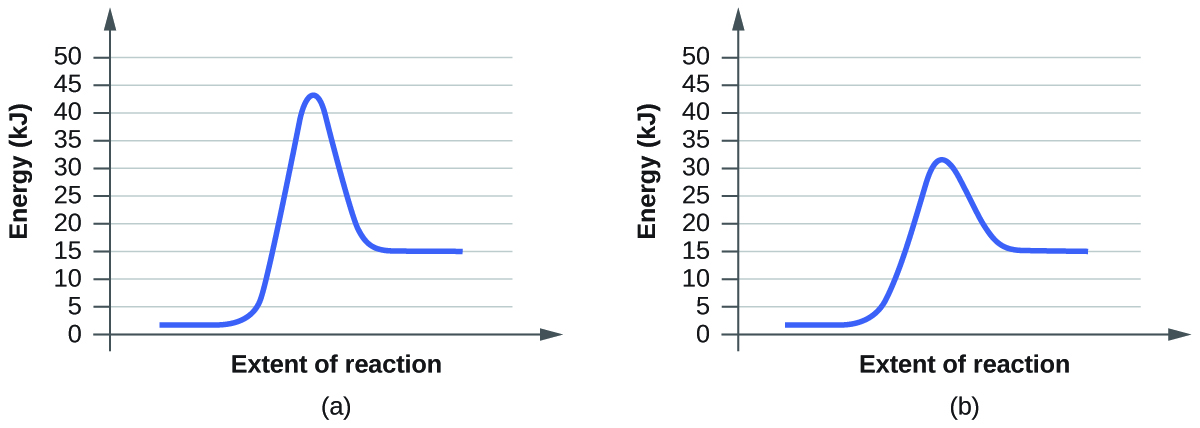 <-(Pair 2
<-(Pair 2
Answer:
The (B) graph of both pair 1 and pair 2 are catalyzed.
Explanation:
Let's define a couple necessary terms to understand the questions.
Reaction diagram- This can be thought of as a picture of how a chemical reaction proceeds. It shows the amount of energy needed to complete a ...
Catalysis- A catalyst is a substance that increases the reaction rate of a chemical reaction without being consumed in the process. It increases the rate of a reaction by lowering the activation energy (the energy it takes to go over the hill), but NOT the total change in energy (products energy - reactants energy = change in energy).
Generally, catalyzed reaction involve a lower activation energy and a change in the reaction mechanism. None of these graphs clearly depict any changes in transition state or intermediates (you can tell because there is a lack of multiple energy troughs and peaks), so only the difference in activation energy can be used to determine whether or not the reaction is catalyzed. Activation energy is the initial energy it takes to cause the chemical reaction to occur; this is represented by the first peak in each graph. A catalyzed reaction has a lower activation energy than the original reaction seen by a smaller bump on the graph.
To clarify, the (B) graph in pair one only requires 20 KJ of energy to start the reaction while the (A) graph needs almost 25 KJ. The (B) graph in pair 2 needs about 32 KJ of energy which is significantly lower compared to (A) about 44 KJ.
Section 17
Q17.1.10
Why must the charge balance in oxidation-reduction reactions?
Answer/Explanation:
The charges should balance in oxidation-reduction reactions because (1) the electrons given off by the oxidation half reaction are taken up by the reduction half reaction and (2) the electrons given off or absorbed affect the charge of the element so that each half reaction is also balanced. These two half reactions occur simultaneously to produce no net change in charge.
For example, take the reaction 2 Mg (s) + O2 (g) → 2 MgO (s).
The half reactions are as follows:
2 Mg → 2 Mg2+ + 4 e-
4 e- + O2 → 2 O2-
As you can see, each half-reaction is balanced within itself and the resulting electrons needed to balance the half reactions cancel each other. When the half-reactions are summed, the overall reaction is 2 Mg (s) + O2 (g) → 2 MgO (s).
Section 19
Q19.1.9
Why is the formation of slag useful during the smelting of iron?
Answer/Explanation:
Slag is useful for smelting Fe2O3 to Fe for a variety of reasons. It is a floating layer above the molten iron and it may contain CaSiO3, mixed oxides, sulfur, phosphorous, and aluminum because natural irons contain contamination. These impurities that form slag are generally less dense than iron (55.85 g/mol) which is why they float and can be easily separated from the iron during processing. This slag is also important because it forms a barrier that prevents the molten iron from oxidizing in the presence of oxygen (or reacting to oxygen in the air), which would turn the iron back to Fe2O3 thus making the whole point of smelting pointless.
Q19.3.1
Determine the number of unpaired electrons expected for [Fe(NO2)6]3−and for [FeF6]3− in terms of crystal field theory.
Answer/Explanation:
1. Determine the main cations involved to find how many d-electrons are involved in the complexes. Use the oxidation number of the cation and count how many electrons are removed.
[Fe(NO2)6]3− and [FeF6]3− both have the Fe3+ cation, which has 5 d-electrons.
Because the normal Fe has 6 d-orbital electrons and 2 s-orbital electrons and the complexes have Fe with 3+ oxidation number, there are 3 fewer electrons in Fe3+ than Fe, but the removal process for electrons must follow certain rules. Electrons must be removed from higher energy levels before the more stable lower energy levels. Iron has the orbital notation of [Ar] 3d64s2 which means that the electrons from the 4s must be taken first because they occupy a higher energy than the 3d orbital when taking away electrons. Thus, we are left with [Ar]3d5.
2. Figure out what the geometry of the complex is with the ligands. In this case, they are both octahedral because there are 6 ligands attached to the metal cation. An octahedral geometry means the following arrangement of electrons is used:
 (drawn with MS Paint)
(drawn with MS Paint)
3. Ligands each have a strength that affect how electrons are placed on the energy splitting field. Strong field ligands cause the low spin to be favored (electrons placed in the t2g first), while weak field ligands favor the high spin (electrons each occupy one space on the t2g and eg before pairing up). The strength of a ligand is determined by the spectrochemical series and it lists F- as a weak ligand and NO2- as a strong ligand.
I− < Br− < S2− < SCN− < Cl− < NO3− < N3− < F− < OH− < C2O42− < H2O
< NCS− < CH3CN < py < NH3 < en < bipy < phen < NO2− < PPh3 < CN− < CO
For this problem, the arrangement of the electrons are as followed:
*Note: Electrons can only fill spaces up one at a time and usually do not double up unless a strong ligand is involved.
 (drawn with MS Paint)
(drawn with MS Paint)
4. Finally, the number of unpaired electrons can be found. [Fe(NO2)6]3− has 1 unpaired electron and for [FeF6]3− has 5 unpaired electrons.
Section 20
Q20.3.13
For each galvanic cell represented by these cell diagrams, determine the spontaneous half-reactions and the overall reaction. Indicate which reaction occurs at the anode and which occurs at the cathode.
- Zn(s)∣Zn2+(aq) ∥ H+(aq)∣H2(g), Pt(s)
- Ag(s)∣AgCl(s)∣Cl−(aq) ∥ H+(aq)∣H2(g)∣Pt(s)
- Pt(s)∣H2(g)∣H+(aq) ∥ Fe2+(aq), Fe3+(aq)∣Pt(s)
Answer:
- reduction: 2H+(aq) + 2e− → H2(aq); cathode;
oxidation: Zn(s) → Zn2+(aq) + 2e−; anode;
overall: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(aq)
- reduction: AgCl(s) + e− → Ag(s) + Cl−(aq); cathode;
oxidation: H2(g) → 2H+(aq) + 2e−; anode;
overall: AgCl(s) + H2(g) → 2H+(aq) + Ag(s) + Cl−(aq)
- reduction: Fe3+(aq) + e− → Fe2+(aq); cathode;
oxidation: H2(g) → 2H+(aq) + 2e−; anode;
overall: 2Fe3+(aq) + H2(g) → 2H+(aq) + 2Fe2+(aq)
Explanation:
First, we need to define a few terms.
- Reduction- This component gains the electrons. It is also considered the oxidizing agent.
- Oxidation- This component loses electrons and is known as the reducing agent.
- Anode- The oxidation reaction occurs on this electrode. In this case, galvanic cells have negatively charged anodes
- Cathode- The reduction reaction occurs on this electrode. In galvanic cells, the cathode is positively charged.
- Inert electrode- An electrode that does not participate chemically, but serves as a source or sink for electrons. Platinum is a good example of this.
- These problems can all be approached the same way, so the first step is to determine the half reactions involved. The half reactions should only contain one element and keep the reactants on the left and products on the right of the arrow. This should result in two different half reactions.
Ex. 1) Zn(s)∣Zn2+(aq) ∥ H+(aq)∣H2(g), Pt(s)
This is written in cell notation, which makes it a lot easier to determine the half reactions because they are grouped for you already. The Pt(s) is not included because it is an inert electrode and does not chemically affect the reaction.
Zn(s) → Zn2+(aq)
H+ → H2(g)
2. Balance the half reactions by (1) balancing the number of elements themselves so that they are stoichiometrically equal [DO NOT change subscripts like the "2" in H2] and (2) balancing the charges using electrons which have a -1 charge so that both sides have the equivalent charge. This results in two different, but now balanced half reactions.
You first need to balance the elements before the charges.
Zn(s) → Zn2+(aq)
2H+ → H2(g)
Next, balance the charges by adding electrons to one end of each half reaction in order to make the charge match on both sides.
Zn(s) → Zn2+(aq) + 2e-
2e-+ 2H+ → H2(g)
3. It should be clear now on which half reaction is the oxidation reaction/anode side and which is the reduction/cathode side (see definitions above).
Zn(s) → Zn2+(aq) + 2e- : oxidation reaction/anode side because the Zn(s) loses electrons to make Zn2+
2e-+ 2H+ → H2(g) : reduction/cathode side because the H+ needs to gain electrons to make the neutral H2(g)
NOTE: It is also useful to know that in cell notation the anode is always on the left and the cathode is always on the right side of the double bar.
4. Make sure that the electrons on both reactions can cancel one another out before adding the two reactions together to produce the overall reaction. If the electrons do not cancel, find a common coefficient that can be multiplied to either reaction to make the electron amounts cancel. Remember that multiplying the coefficient across the reaction also affects the coefficients for the elements.
In this case, the reaction works out perfectly because there are 2 electrons on opposite ends of each other, so the two half reactions can be added together.
2e-+ 2H+ + Zn(s) → Zn2+(aq) + H2(g) + 2e-
This is the finished overall chemical redox reaction.
Q20.5.26
Ideally, any half-reaction with E° > 1.23 V will oxidize water as a result of the half-reaction O2(g) + 4H+(aq) + 4e− → 2H2O(l).
- Will FeO42− oxidize water if the half-reaction for the reduction of Fe(VI) → Fe(III) is FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O; E° = 1.9 V?
- What is the highest pH at which this reaction will proceed spontaneously if [Fe3+] = [FeO42−] = 1.0 M and PO2= 1.0 atm?
Answer:
Part 1.
a) To figure out whether or not FeO42− will oxidize water, the half-reactions given should be clarified.
This can be done by rearranging the information given to make it easier to understand the problem.
Reduction/Cathode: FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O; E° = 1.9 V
Oxidation/Anode: 2 H2O → O2 + 4H+ + 4e-; E° = 1.23 V
b) The question is only asking if FeO42− will oxidize water, so the best way to determine whether a reaction is likely to happen or not is to determine its spontaneity. In electrochemical reactions such as this one, the cell potential can be used. Cell potential (E°cell) is found by the following formula: E°cell = E°cathode - E°anode
Standard reduction potential is an intensive property, so changing the stoichiometric coefficient in a half reaction does not affect the value of the standard potential. As a result, you do not need to balance the equation to solve for cell potential.
c) Find the overall cell potential using E°cell = E°cathode - E°anode and if E°cell is positive then the reaction is spontaneous or a favored reaction. If E°cell is negative then the reaction is not spontaneous.
E°cell = 1.9 V - 1.23 V = 0.67 V which is positive, so the reaction FeO42−(aq) will oxidize water.
Part 2.
a) We know that a change in pH is a change in the H+ concentration, so we are looking for the value where H+ is as large as it can be while the reaction remains spontaneous. We also know [Fe3+] = [FeO42−] = 1.0 M and PO2= 1.0 atm, which means the reaction takes place in standard conditions. The only formula that combines concentrations with cell potentials at standard conditions is the Nerst equation.
\[E = E^o - \dfrac{0.0592\, V}{n} \log Q \tag{Nernst Equation @ 298 K}\]
298K is used because we are not given a temperture and the reaction takes place at standard conditions.
b) Figure out the overall balanced reaction so that the variables can be defined.
Original:
Reduction/Cathode: FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O; E° = 1.9 V
Oxidation/Anode: 2 H2O → O2 + 4H+ + 4e-; E° = 1.23 V
Stoichiometrically balance the electrons:
4 (FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O) : multiply by 4
3 (2 H2O → O2 + 4H+ + 4e-): multiply by 3
Result:
4 FeO42−(aq) + 32 H+(aq) + 12e− → 4Fe3+(aq) + 16H2O
6 H2O → 3O2 + 12H+ + 12e- : the 12 electrons on both sides cancel
Overall: Add the half reactions together and subtract species that exist on both sides
4FeO42−(aq) + 20 H+(aq) → 4Fe3+(aq) + 10 H2O (l) + 3O2(g)
c) Define relevant variables.
E = 0, because we want to setup the equation so that the result is a positive value (spontaneous)
E° = this has been calculated in part 1 = 0.67 V
n = number of electrons involved= 12 e- in this case
Q = reaction quotient (product/reactant concentrations)
= [Fe3+]4[O2]3/ [FeO42−]4[H+]20
= [1.0 M]4[1 atm]3/ [1.0 M]4[H+]20
= 1/[H+]20
d) Plug into formula.
\[E < E^o - \dfrac{0.0592\, V}{n} \log Q \]
0 < 0.67 V - (0.0592 V/ 12)* log(1/[H+]20)
0.67 V < (0.0592 V/ 12)* log(1/[H+]20)
(0.67 V)*(12/ 0.0592 V) < log(1/[H+]20)
10^(135.81) < 10^{log(1/[H+]20)}
(6.469 E135) < (1/[H+]20)
[H+]20 < [1/(6.469 E135)]
[H+] < [1/(6.469 E135)]^(1/20)
[H+] < 1.62 E-07
e) Based on the results, [H+] < 1.62 E-07, we know that the [H+] must be less than 1.62 E-07 M to fit the criteria of spontaneity. The calculation for pH can now be done using the formula pH = -log(H+).
pH = -log(1.62 E-07)
pH = 6.79
The highest pH is pH = 6.79
Section 21
Q21.4.27
Write a balanced equation for each of the following nuclear reactions:
- bismuth-212 decays into polonium-212
- beryllium-8 and a positron are produced by the decay of an unstable nucleus
- neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
- strontium-90 decays into yttrium-90
Answer:
1. bismuth-212 decays into polonium-212
Result:
\[ ^{212}_{83}\text{Bi}\rightarrow ^{212}_{84}\text{Po} +^{0}_{-1}\beta \]
2. beryllium-8 and a positron are produced by the decay of an unstable nucleus
Result:
\[ ^{8}_{5}\text{B}\rightarrow ^{8}_{4}\text{Be} +^{0}_{1}\beta \]
3. neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
Result:
\[ ^{238}_{92}\text{U} +^{1}_{0}\text{n}\rightarrow ^{239}_{93}\text{Np} +^{0}_{-1}\beta\]
\[^{239}_{93}\text{Np}\rightarrow ^{239}_{94}\text{Pu} +^{0}_{-1}\beta \]
4. strontium-90 decays into yttrium-90
Result:
\[ ^{90}_{38}\text{Sr}\rightarrow ^{90}_{39}\text{Y} +^{0}_{-1}\beta \]
Explanation:
Note: The 4 possible particles
- Alpha 42He
- Beta
- Electrons 0-1e
- Positrons 01p
- Gamma 00g
- Neutrons 10n
To approach these questions, it is useful to translate the information given into "skeleton equations" to see which part is missing. This means to only write the given information in the equation form without attempting to balance anything yet. Doing this step makes complicated equations easier to understand.
ex. neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
This looks complicated but it can be broken down into the following form:
The first part to do is "neptunium-239 forms from the reaction of uranium-238 with a neutron". "From" indicates that uranium-238 and a neutron are responsible for making neptunium-239.
\[ ^{238}_{92}\text{U} +^{1}_{0}\text{n} \rightarrow ^{239}_{93}\text{Np} \]
This equation is not yet balanced, so it can be balanced by figuring out what must be added so that the mass and charge on both sides are equal.
One one side--
Uranium: mass of 238 and atomic number (positive charge) of 92
Neutron: mass of 1 and no charge
On the other side--
Neptunium: mass of 239 and atomic number (proton charge) of 93
Missing particle: ?
So in terms of mass, the missing mass is ...
238 + 1 = 239 + x
0 amu= x
In terms of charge, the missing charge is...
92 + 0 = 93 + y
-1 = y
The only particle with mass 0 and charge -1 is a beta particle (also known as an electron). The only logical placement for this particle is on the neptunium side where the answer is the following:
\[ ^{238}_{92}\text{U} +^{1}_{0}\text{n}\rightarrow ^{239}_{93}\text{Np} +^{0}_{-1}\beta\]
The second part of the question is "then spontaneously converts into plutonium-239". This means that the neptunium breaks down further into plutonium. We know that neptunium has the mass of 239 and atomic number of 93. Plutonium has the mass of 239 and atomic number of 94. The skeleton equation formed is the following:
\[^{239}_{93}\text{Np}\rightarrow ^{239}_{94}\text{Pu} \]
Now the missing particle can be determined. We also know that neptunium is broken down, so no other particle can be on the neptunium side.
Mass: 239 = 239 + x
0 amu = x
Charge: 93 = 94 + y
-1 = y
Once again the missing particle is a beta particle, so the balanced equation is
\[^{239}_{93}\text{Np}\rightarrow ^{239}_{94}\text{Pu} +^{0}_{-1}\beta \]
Finally, repeat these steps for each question.

