Extra Credit 41
- Page ID
- 82903
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.1
![]()
Which member of each pair of metals is more likely to corrode (oxidize)?
- Mg or Ca
- Au or Hg
- Fe or Zn
- Ag or Pt
S17.6.1
Solution:
Oxidation is the loss of electrons or the gain in oxidation state, thus the approach one must take is to pick the element in each pair that is more likely to give up an electron easily compared to the other one. One way to analyze this is to choose the least electronegative element since it is less likely to attract electrons to it, therefore is more easily oxidized since it will be more willing to give up an electron. Electronegativity increases from left to right across the periodic table and up each column or group.
As a result, the corresponding answers are:
1. Ca
2. Au
3. Fe
4. Pt
because these are elements in each pair that are more likely to give up an electron, and thus, is more likely to be oxidized or corrode.
reviewed and modified by Heather Hu
Q12.3.4
How much and in what direction will each of the following affect the rate of the reaction: CO(g) + NO2(g) ⟶ CO2(g) + NO(g) if the rate law for the reaction is rate= k[NO2]2?
- Decreasing the pressure of NO2 from 0.50 atm to 0.250 atm.
- Increasing the concentration of CO from 0.01 M to 0.03 M.
S12.3.4
Solution:
1. Since this system is not at equilibrium and is only written in the forward direction, we can assume that this direction is favored or for the sake of this question, the forward reaction is the direction of the reaction. In addition, the given rate law is rate = k[NO2]2, thus showing that this system is dependent on NO2 only. And the overall reaction order is 2. Therefore, by halving the original pressure would result in a rate reduction by a factor of 4 since the quantity is squared.
ex. rate = k[0.5]2=0.25k
new conditions: rate = k[.25]2 = .0625k which is 1/4th the value of the original, and thus showing that by decreasing the pressure of NO2 by a half value will cause a reduction in the rate by a factor of four.
2. The change in concentration of [CO] is irrelevant since the rate is dependent only on the reactant NO2 as shown and supported by the rate law:
rate = k[NO2]2. Therefore, there would be no change in direction or rate if the concentration of [CO] was changed.
reviewed and modified by Heather Hu
Q12.5.13
Hydrogen iodide, HI, decomposes in the gas phase to produce hydrogen, H2, and iodine, I2. The value of the rate constant, k, for the reaction was measured at several different temperatures and the data are shown here:
| Temperature (K) | k (M−1 s−1) |
|---|---|
| 555 | 6.23 × 10−7 |
| 575 | 2.42 × 10−6 |
| 645 | 1.44 × 10−4 |
| 700 | 2.01 × 10−3 |
What is the value of the activation energy (in kJ/mol) for this reaction.
S12.5.13
Solution:
Given: Temperature in Kelvin(K) as well as the rate constant k. Note: This is a Second Order Reaction since the units of k are in M-1s-1, and when multiplied with a squared concentration, typical of a second order reaction, it will result in the proper units of rate (M/s).
ln(K2/K1) = (-Ea/R)[(1/T2)-(1/T1)]
Where the rate constants K2 and K1 correspond with the temperatures (Kelvin) T2 and T1, R is the ideal gas constant (R=8.3145J/mol-K), and Ea is the activation energy in J/mol.
Solve:
Plug in two values for each rate constant and temperature, ensuring that the temperature corresponds to the rate constant.
Here we used 500K with 6.23 x 10-7 and 700K with 2.01 x 10-3 , where K1 and T1 are the first pair of values and K2 and T2 are the second set.
ln(2.01 x 10-3/ 6.23 x 10-7) = (-Ea/8.3145)[(1/700)-(1/500)]
Once simplifying for the activation energy (Ea) by basic algebra, one finds the value to be:
179979.1 J/mol or 180 kJ/ mol.
reviewed and modified by Heather Hu
Q21.4.8
The following nuclei do not lie in the band of stability. How would they be expected to decay? Explain your answer.
- \(\ce{^{34}_{15}P}\)
- \(\ce{^{239}_{92}U}\)
- \(\ce{^{38}_{20}Ca}\)
- \(\ce{^{3}_{1}H}\)
- \(\ce{^{245}_{94}Pu}\)
S21.4.8
Solution:
The belt of stability below shows the combination of neutrons to protons of nuclei that are stable. However, all elements above Z = 83 are considered radioactive and mostly emit alpha particles. The combinations of neutrons to protons of nuclei that fall below the belt of stability, and have a Z < 83 value, are considered to have positron emission denoted by ß+. In addition, the ratio of neutrons to protons that are greater than or lie above the belt of stability are likely to have ß- decay.

With this information in mind, for further stability of each element, one can now properly analyze each of the following problems and determine which type of decay the individual nuclei will undergo according to their placement with respect to the belt of stability.
1. Above the belt of stability, yields ß- decay.
2. Has a Proton count higher than 83, thus would undergo α decay.
3. Below the belt of stability, yields ß+ emission.
4. Above the belt of stability, yields ß- decay.
5. Has a Proton count higher than 83, thus would undergo α decay.
Agreed. reviewed and modified by Heather Hu
Q20.2.12
![]()
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation; then write the complete ionic equation for the reaction.
- A few drops of NiBr2 are dropped onto a piece of iron.
- A strip of zinc is placed into a solution of HCl.
- Copper is dipped into a solution of ZnCl2.
- A solution of silver nitrate is dropped onto an aluminum plate.
S20.2.12
Solution:
An activation series is a series of metals that are organized from metals that are most reactive or likely to be oxidized from the top of the list to the bottom. The metal at the top of the list will replace a metal below it in a displacement reaction.
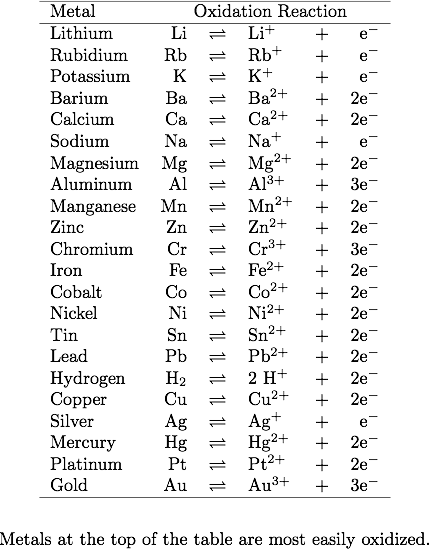
1. A reaction should occur on the surface of the Iron where the solution of NiBr2 was dropped because Iron is more reactive and higher up on the activation series than Nickel. Since Bromine is a halogen and is missing one valence electron it has an oxidation state of 1-. In effect, with two Bromines with a 1- oxidation state, that means that Nickel and Iron must have oxidation states of 2+ to even to even out the charges. The resulting net ionic and ionic equation is:
Net Ionic Equation: Fe2+ (s) + Br2 (aq) → FeBr2 (aq)
Ionic Equation: Fe2+ (s) + NiBr2 (aq) → FeBr2 (aq) + Ni2+ (aq)
2. A reaction should proceed between the Zinc strip and the HCl solution since Zinc is more reactive than Hydrogen according to the activation series. The oxidation state of Hydrogen is always 1+, thus Chlorine must have an oxidation state of 1- as a result to obtain an overall molecule charge of 0. In addition, Zinc has an oxidation state of 2+ as indicated by the table above, and results in the following equations:
Net Ionic Equation: Zn2+ (s) + 2Cl- (aq) → ZnCl2 (aq)
Ionic Equation: Zn2+ (s) + 2HCl (aq) → ZnCl2 (aq) + 2H+(aq)
3. No reaction will occur since Zinc is more reactive than Copper and thus will stay bonded in its current state with Chlorine due to how much more it is to be oxidized.
4. A reaction would occur here between the silver nitrate solution and the aluminum plate because Aluminum is more reactive than silver according to the activity series, thus is oxidized more and is more likely to be attracted to the electronegative nitrate compound. The nitrate compound (NO32-) has an overall oxidation state of 2- and therefore the silver must have an oxidation state of 2+ to balance that charge. As well, if aluminum is replacing silver it also must have an oxidation state of 2+ such that the resulting net ionic and ionic equations are:
Net Ionic Equation: Al2+ (s) + NO22- (aq) → Al(NO2) (aq)
Ionic Equation: Al2+ (s) + Ag(NO2) (aq) → Al(NO2) (aq) + Ag (aq)
Agreed. reviewed and modified by Heather Hu
Q20.5.7
![]()
Occasionally, you will find high-quality electronic equipment that has its electronic components plated in gold. What is the advantage of this?
S20.5.7
![]() Solution:
Solution:
The standard reduction potential(SRP) is a measure of how well a substance is reduced in their reduction reaction. The more negative the SRP is, the better the element as a reducing agent. Below has a table of standard reduction potentials of certain elements and molecules, with positive values (better at acting as an oxidizing agent ) at the top and more negative values (better at acting as a reducing agent) toward the bottom.
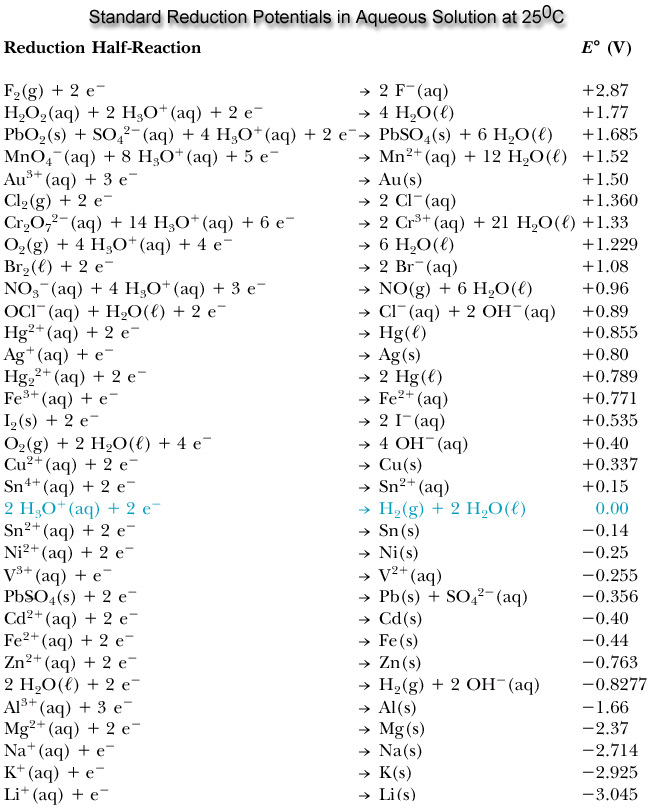
In effect, it can be seen how Gold is higher on the reduction potential table. Thus, Gold is more likely to undergo reduction rather than oxidation while Zinc, for example, is more likely to undergo oxidation rather than reduction due to Zinc's more negative SRP. Therefore, this is why one might find expensive and high-quality electrical equipment coated in Gold due to its small likelihood to corrode as it is hard to oxidize ability to not corrode (become oxidized and lose electrons) and maintain its chemical characteristics because of its high reduction potential compared to many metals found in electronic equipment.
Reviewed and modified by Heather Hu
Q24.6.3
![]()
Will the value of Δo increase or decrease if I−ligands are replaced by NO2−ligands? Why?
S24.6.3
Solution:
Yes, the value of Δo will increase since one is replacing a weak field ligand(I), or a ligand with a small Δo value, with a ligand (NO2) that is a strong field ligand with respect to the original ligand (I). Therefore, this indicates that it is a ligand that has a larger Δo value that is replacing the weaker one. Because the Δo is the crystal field splitting of a ligand, the larger Δo corresponds with larger energy as well as stronger field splitting. So the Value of Δo will increases if I- are replaced by NO2- ligands.
Reviewed and modified by Heather Hu
Q12.1
![]()
The following data were obtained for the reaction of methane with oxygen:
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
|
time(min) |
[CH4] (mol/L) |
[CO2] (mol/L) |
|---|---|---|
|
30 |
0.015 |
? |
|
20 |
0.020 |
? |
|
10 |
0.030 |
0.020 |
|
0 |
0.050 |
0 |
- How many moles of CO2 are produced for each mole of CH4 that is used up?
- What concentration of CH4 is used up after 10 minutes?
- What is the concentration of carbon dioxide produced after 20 minutes?
- Write an equation for reaction rate in terms of Δ[CO2] over a time interval.
- What is the reaction rate for the formation of carbon dioxide between 10 and 20 minutes?
- What is the average reaction rate between 0 and 30 minutes?
- Write an expression for reaction rate relating Δ[O2] to Δ[CO2].
- At what rate is O2 used up between 10 and 20 minutes?
S12.1
Solution:
1. According to the balanced reaction, the stoichiometric ratio between CO2 and CH4 is 1:1 since that is the coefficient in the reaction, thus for every 1 CH4 reacted, 1 CO2 is produced.
2. The initial concentration of CH4 is indicated by the given table and says at time = 0 that the initial concentration is .050M and at 10 minutes it is then .030M. Thus, the concentration of CH4 used up after 10 minutes is equal to .050M - .030M = .020M.
3. Since the stoichiometric ratio between CO2 and CH4 is 1:1, the concentration of CO2 produced is directly proportional to the CH4 reacted. The CH4 reacted after 20 minutes is equal to .050M - .020M = .030M, thus the concentration of CO2 after 20 minutes is .030M.
4. An equation for the reaction rate in tems of [CO2] over a time interval is:
Δ[CO2] = d[CO2 ]/dt = ([CO2]Final - [CO2]Initial)/(timeFinal - timeInitial)
where dt is in respect to time t(minutes).
5. The reaction rate for the formation of carbon dioxide between 10 and 20 minutes is :
= (.030M - .020M)/(20 Minutes - 10 Minutes)
= .010M/10 Minutes
= .001M/Min.
6. The average reaction rate from 0 to 30 minutes is equal to:
Δ[CO 2] = -Δ[CH4]
= (.015M - .050M)/(30 Mins. - 0 Mins.)
= -.00117M/Min.
7. The rate of change of Oxygen over time t in minutes is equal to Δ[O2] which is furthermore equal to:
Δ[O2] = -(1/2)(d[O2]/dt) = d[CO2]/dt = Δ[CO2]
where dt is in respect to t time (Minutes). The -1/2 is to take into account the stoichiometric ratio between O2 and CO2 in the rate.
8. The rate O2 is used up between 10 and 20 minutes is equal to
Δ[O2]20mins-10mins = -(1/2)[([O2]20mins - [O2]10mins)/(20mins - 10mins)] = ([CO2]20mins - [CO2]10mins)/(20mins-10mins) = Δ[CO2]
= -(1/2)[([O2]20mins - [O2]10mins)/(20mins - 10mins)] = ([.030M]20mins - [.020M]10mins)/(20mins-10mins)
= -(1/2)[([O2]20mins - [O2]10mins)/(20mins - 10mins)] = .010M/10mins
= [([O2]20mins - [O2]10mins)/(10mins)] = -2(.0010M/mins)
= Δ[O2]20mins-10mins = -.002M/mins
Agreed. Reviewed and modified by Heather Hu

