Extra Credit 32
- Page ID
- 82893
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.4.5
Use the data in Table P1 to determine the equilibrium constant for the following reactions. Assume 298.15 K if no temperature is given.
- \(\ce{AgCl}(s)⇌\ce{Ag+}(aq)+\ce{Cl-}(aq)\)
- \(\ce{CdS}(s)⇌\ce{Cd^2+}(aq)+\ce{S^2-}(aq) \hspace{40px} \textrm{at 377 K}\)
- \(\ce{Hg^2+}(aq)+\ce{4Br-}(aq)⇌\ce{[HgBr4]^2-}(aq)\)
- \(\ce{H2O}(l)⇌\ce{H+}(aq)+\ce{OH-}(aq) \hspace{40px} \textrm{at 25 °C}\)
Needed equation: \[E^{_{cell}^{\circ }}= \frac{RT}{nF}lnK\]
Variables:
- R is a constant that is equal to \(8.3145 J\cdot mol^{-1}\cdot K^{-1}\)
- T is the temperature in Kelvin (this is stated in the problem)
- F is Faraday's Constant which is equal to \(96485 J\cdot V^{-1}\cdot mol^{-1}\)
- n is the number of electrons transferred between oxidants and reductants. We will use the redox half reactions to find n.
- \(E^\circ_{\ce{cell}}\) (the standard potential of the reaction) is given by the equation \(E_{cell}^{\circ}=E_{cath}^{\circ}-E_{anode}^{\circ}\). The standard potential of the cathode (the reduction half reaction) and the anode (the oxidation half reaction) can be found in Table P1.
- K is the equilibrium constant that we are solving for.
Solve:
a.) First find the redox half reactions where chlorine is being reduced and silver is being oxidized. We know this because Ag goes from an oxidation number of 0 to +1 meaning it lost an electron (oxidation) so Cl must have been reduced in order to accept the electron. The following half reactions and their standard redox potentials were found from Table P1.
Cathode/Reduction half reaction: \(AgCl(s)+e^{-}\rightarrow Ag(s)+Cl^{-}\) \(E_{cath}^{\circ}=.2223\)
Anode/Oxidation half reaction: \(Ag(s)\rightarrow Ag^{+}(aq)+e^{-}\) \(E_{anode}^{\circ}=.7996\)
From this we can find \(E_{cell}^{\circ}=.2223-.7996=-.5773\) and that n=1 since 1 electron is being transferred (If you had an unequal number of electrons between the equations, you'd scale the equations as you would if you were balancing and then would use the coefficient of electrons in the properly scaled half reactions). Also T=298.15K as stated in the problem. We now have \(E_{cell}^{\circ}\), T, n, F (constant), and R(constant). By pluggin in our variables we get \(k=1.7\times 10^{-10}\).
Following this same method for b, c, and d you get
b.)
- T=377
- anode/oxidation: \(Cd^{2+}+2e^{-}\rightarrow Cd(s))\) E°anode=-0.4030
- cathode/reduction: \(S(s)+2e^{-}\rightarrow S^{2-}\) E°cath=-0.4070
- E°cell=-.4070+.4030=-.004
- n=2 (2 electrons being transferred)
\(k=2.6\times 10^{-21}\)
c.)
- T=298.15K
- anode/oxidation: \(Br_{2}+2e^{-}\rightarrow 2Br^{-}\) E°anode=1.087
- cathode/reduction: \(2Hg^{2+}+2e^{-}\rightarrow Hg_{2}^{2+}\) E°cath=.911
- E°cell=.911-1.087=-.176
- n=2
\(k=1.1\times 10^{-6}\)
d.)
- T(K)=T(°C)+273.15=25+273.15=298.15K
- anode/oxidation: \(2H^{+}+2e^{-}\rightarrow H_{2}(g)\) E°anode=0
- cathode/reduction: \(H_{2}O+e^{-}\rightarrow \frac{1}{2}H_{2}(g)+OH^{-}\) E°cath=-.828
- E°cell=-.828
- n=2 (even though one of the equations has 1 electron, you'd have to scale it up to 2 in order for the equations to balance)
\(k=1.0\times 10^{-26}\)
Q12.1.5
A study of the rate of the reaction represented as 2A⟶B gave the following data:
| Time (s) | 0.0 | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 35.0 |
|---|---|---|---|---|---|---|---|
| [A] (M) | 1.00 | 0.952 | 0.625 | 0.465 | 0.370 | 0.308 | 0.230 |
- Determine the average rate of disappearance of A between 0.0 s and 10.0 s, and between 10.0 s and 20.0 s.
- Estimate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus [A]. What are the units of this rate?
- Use the rates found in parts (a) and (b) to determine the average rate of formation of B between 0.00 s and 10.0 s, and the instantaneous rate of formation of B at 15.0 s.
Equations: \(\frac{-\bigtriangleup A}{\bigtriangleup time}\) and Rate=\(\frac{-\bigtriangleup A}{2\bigtriangleup time}=\frac{\bigtriangleup B}{time}\)
Solve: 1.)The change in A from 0s to 10s is .625-1=-.375 so \(\frac{-\bigtriangleup A}{\bigtriangleup time}\)=.375/10= 0.0374 M/s
Similarly, the change in A from 10 to 20 seconds is .370-.625=-.255 so \(\frac{-\bigtriangleup A}{\bigtriangleup time}\)=.255/20-10= 0.0255M/s
2.) We can estimate the rate law graphing the points against different order equations to determine the right order.
Zero Order: \[\frac{d[A]}{dt}=-k\] \[\int_{A_{\circ}}^{A}d[A]=-k\int_{0}^{t}dt\] \[[A]=-kt+[A_{\circ}]\]
First Order: \[\frac{d[A]}{dt}=-k[A]\] \[\int_{A_{\circ}}^{A}\frac{d[A]}{[A]}=-kdt\] \[Ln(A)=-kt+Ln(A_{\circ})\]
Second Order: \[\frac{d[A]}{dt}=-k[A]^{2}\] \[\int_{A\circ}^{A}\frac{d[A]}{[A]^{2}}=-k\int_{0}^{t}dt\]
\[\frac{1}{[A]}=kt+\frac{1}{[A_{\circ}]}\]
Now that we have found the linear from of each order we will plot the points vs an [A] y-axis, a Ln(A) y-axis, and a 1/[A] y-axis. whichever of the plots has the most linear points will give us a good idea of the order and the slope will be the k value.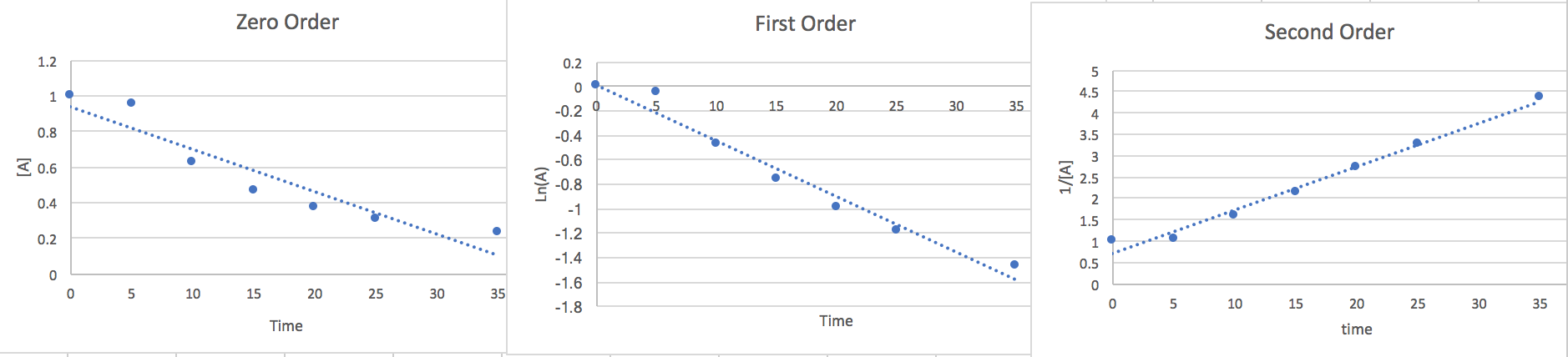
Here we notice that the second order is most linear so we conclude the Rate to be.. \[\frac{-d[A]}{2dt}=k[A]^{2}\] At 15 seconds [A]=.465 and from the slope of the graph we find k=.116.so if we plug this data in and multiply both sides by 2 to get rid of the 2 in the denominator on the left side of the equation we find that the rate of disappearance of A is .05 M/s where the units are equivalent to [mol*L-1*s-1]
3.) Using the equation \(\frac{-\bigtriangleup A}{2\bigtriangleup time}=\frac{\bigtriangleup B}{time}\) we divide the rates in part a and b in half to get .0188 M/s from 0 to 10 seconds and .025 M/s for the estimated instantaneous rate at 15s.
Q12.5.3
What is the activation energy of a reaction, and how is this energy related to the activated complex of the reaction?
Solution:

Activation energy is the energy barrier that must be overcome in order for a reaction to occur. To get the bonds into a state that allows them to break, the molecule must be contorted (deformed, or bent) into an unstable state called the transition state. The transition state is a high-energy state, and some amount of energy – the activation energy – must be added in order for the molecule reach it. Because the transition state is unstable, reactant molecules don’t stay there long, but quickly proceed to the next step of the chemical reaction.This is related to the activated complex in that the energy of the activated complex (structure at maximum energy) minus the energy of the reactants is equal to the activation energy.
Q21.3.7
The mass of the atom \(_{9}^{19}\textrm{F}\) is 18.99840 amu.
- Calculate its binding energy per atom in millions of electron volts.
- Calculate its binding energy per nucleon.
Equations: \(9p+10n\rightarrow _{9}^{19}\textrm{F}\) and \(\bigtriangleup E=\bigtriangleup mc^{2}\)
c=3.00E8
\(_{9}^{19}\textrm{F}\): We know that F has 9 protons since its atomic number is 9 as denoted by the lower subscript. To find the number of neutrons, we subtract the number of protons from its atomic mass as denoted by the upper subscript (19 in this case) to get 19-9=10 neutrons.
mass of (n)eutron=1.008664 amu
mass of (p)roton=1.007276 amu
mass of \(_{9}^{19}\textrm{F}\)=18.99840 amu
Solve: a.) Mass= (mass of proton x number of protons) + (mass of neutrons x number of neutrons)
mass of products - mass or reactants = \(\bigtriangleup m\) = 18.9984-[(9)1.007276+(10)1.008664]=-.153724 amu
\(-.153724 amu*\frac{1.66054*10^{-27} kg}{1 amu}=-2.5526485*10^{-28}kg\)
\(\bigtriangleup E\)=(\(-2.5526485*10^{-28}kg\))(\(3.00*10^{8})^{2}\)=\(-2.2973837*10^{-11}J*\frac{6.242*10^{12} MeV}{1 J}\)=\(143.4 \frac{MeV}{atom}\) NOTE: you may get slightly different answers if you round too much or if you use the mass of proton from professor Larsens forumula sheet which he give's as 1.0078 amu which will give you an answer of 148 MeV for this problem
b.) \(143.4\frac{MeV}{atom}*\frac{atom}{19 nucleon}\)=7.548 \(\frac{MeV}{nucleon}\)
Q20.2.3
In each redox reaction, determine which species is oxidized and which is reduced:
- Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
- Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l)
- BrO3−(aq) + 2MnO2(s) + H2O(l) → Br−(aq) + 2MnO4−(aq) + 2H+(aq)
How to solve: Determine the oxidation number of each element on the left and right of the equation. If the oxidation number increases from the left side to the right side of the equation then the element is oxidized and if the oxidation number for a species decreases from the left side of the equation to the right then that species is reduced. (An oxidation number is a bookkeeping tool to keep track of the "charge" of the species throughout the reaction). If an element is alone its oxidation number is its charge. If an element is in a species its oxidation number can be found by adding all the oxidation numbers and setting them equal to the total charge. For example: to find the oxidation number of N in HNO3 solve for x in (+1)+x+3(-2)=0. Thus nitrogen's oxidation number is +5. If the species had a total charge you'd set the equation equal to that charge rather than 0. NOTE: O commonly has an oxidation number of -2.
a.) Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Zn: On the left side of the reaction zing has an oxidation number of zero since it has no charge and on the right it has an oxidation number of +2 since it makes the sulfate with a -2 charge neutral as a molecule. Since Zinc's oxidation number goes from 0 to +2 it is oxidized. Zn(s) is oxidized.
H: On the left side H has an oxidation number of +1 since it takes 2 to make the -2 charged sulfate neutral. On the right H has an oxidation number of 0 since it has no charge. Since Hydrogens oxidation number decreases, it is reduced. H2SO4(aq) is reduced.
b.) Using the same process you will find that copper is oxidized since its oxidation number increases from 0 to +2 in the species Cu(NO3)2 (x+2(-1)=0) Cu(s) is oxidized. and nitrogen is reduced since its oxidation number decreases from +5 in the species HNO3 to +4 in the species NO2 (x+2(-2)=0) HNO3(aq) is reduced
c.) Br is reduced since its oxidation number goes from +5 in BrO3− (x+3(-2)=-1) to -1 BrO3- (aq)is reduced and Mn is oxidized since its oxidation number goes from +4 in MnO2 (x+2(-2)=0) to +7 in MnO4− (x+4(-2)=-1) MnO2(s) is oxidized
Q20.4.22
Calculate E°cell and ΔG° for the redox reaction represented by the cell diagram Pt(s)∣Cl2(g, 1 atm)∥ZnCl2(aq, 1 M)∣Zn(s). Will this reaction occur spontaneously?
Solution: What you need to know about cell diagrams is that double lines represent the salt bridge that separates anodes and cathodes (with the anode on the left and the cathode on the right) and that the single lines separate different phases (usually products and reactants). Also, the electrode doesnt have to be made of the substance that is being oxidized or reduced. If there is nothing to "grab onto" such as a gas or liquid, then an inert electrode such as Pt(s) or graphite can be used. From this information we can deduce an equation. The three species participating in the reaction are Cl2, ZnCl2, and Zn(s). Starting on the right side of the cell diagram (which is the cathode/reduction side), we know that Zinc is being reduced and that its oxidation number in ZnCl2(aq) is +2 to balance out the formal charge of the two -1 chlorines and that its oxidation number for Zn(s) is 0 since it's neutral. Therefore the reduction reaction that occurs at the cathode is Zn2++2e-→Zn(s). This implies that ZnCl2 is a reactant and Zn(s) is a product. Now we look at the left side of the cell diagram: The only species we have here is Cl2(g) and we know it is being oxidized since it is on the left. the oxidation number of Cl2 is zero since it is neutral and we know the oxidation number of Cl in ZnCl2 is -1 so we know that this half reaction is Cl22-→Cl2+2e- and that Cl2 must be a product in order for its oxidation number to increase. Therefore the full reaction is: \[ZnCl_{2}(aq)\rightarrow Zn(s)+Cl_{2}(g)\]
We can solve for E°cell=E°cath-E°anode by using the standard redox potentials for our half reactions which can be found in the following link: https://chem.libretexts.org/Reference/Reference_Tables/Electrochemistry_Tables/P1%3A_Standard_Reduction_Potentials_by_Element.
Cathode: Zn2++2e-→Zn(s) E°cath=-0.7618
Anode: Cl2+2e- →2Cl- E°anode=1.396
E°cell=E°cath-E°anode=-0.7618-1.396=-2.1578
We can use this information to find \(\bigtriangleup G^{\circ}\) by using the equation \(\bigtriangleup G^{\circ}=-nFE^{\circ}\)
- Here n equals the number of electrons transferred which is 2 as seen in our half reactions
- F is Faraday's constant which is equal to 96485 J*V-1*mol-1
- E° is our calculated cell potential which was -2.1578
\[\bigtriangleup G^{\circ}=-(2 mol)(96.485 kJ*V^{-1}*mol^{-1})(-2.1578 V)=2,138 kJ\]
Since E°cell<0 and \(\bigtriangleup G^{\circ}\)>0 we know the reaction is non-spontaneous
Q20.9.7
What volume of chlorine gas at standard temperature and pressure is evolved when a solution of MgCl2 is electrolyzed using a current of 12.4 A for 1.0 h?
Reaction: \(MgCl_{2}+2e^{-}\rightarrow Mg+Cl_{2}\)
Equations: \(n_{e}=\frac{It}{F}\) and PV=nRT
Variables:
- \(n_{e}\) is moles of electrons
- I is the current which is given to be 12.4A
- t is the time in seconds which is \(1.0 hr\frac{3600 seconds}{1 hr}\)
- F is Faradays constant which equal 96485 J*mol-2*V-1
- Standard Pressure and Temperature: T=273K P=1 atm
- R is a constant which equals 0.0821 L*atm*mol-1*K-1
Solve: \(n_{e}\)=\(\frac{12.4A*3600s}{96485 J*V^{-1}*mol^{-1}}\)=0.463 moles of electrons
Now convert moles of electrons to moles of chlorine
\(0.463 moles of e^{-1}*\frac{1 mol Cl_{2}}{2 moles of e^{-1}}\)=0.231 moles of Cl2
Now use the ideal gas equation to solve for moles of chlorine.
\[V=\frac{nRT}{P}=\frac{0.231*0.0821*273}{1}=5.2 L\]
Q14.6.5
Before being sent on an assignment, an aging James Bond was sent off to a health farm where part of the program’s focus was to purge his body of radicals. Why was this goal considered important to his health?
Solution: Free radicals are uncharged molecules with an unpaired valence electron. The reason these are so dangerous is because they like to grab electrons from other atoms to fill their own outer shell. This allows them to impair protein function because free radicals readily oxidize proteins and cell membrane which could lead to a loss of function. It was important to purge James Bond of radicals because radicals set off chain reactions of continuously pulling electrons from molecules, which in turn, can damage cells in the body.

