Extra Credit 29
- Page ID
- 82889
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Question 17.4.2
For the ΔG° values given here, determine the standard cell potential for the cell.
- 12 kJ/mol, n = 3
- −45 kJ/mol, n = 1
Answer 17.4.2
- We use this equation for both parts of the problem:
\[Eºcell=\frac{-ΔG°}{nF}\]
- ΔG°=12 KJ, n=3, F=96,485 Coulombs/mole
\[Eºcell=\frac{(-12 KJ/mole)(1000J/KJ)}{(3 mole)(96,485 C/mole)}=-0.0415 V\]
- ΔG°=-45 KJ, n=1, F=96,485 Coulombs/moleWe use the same equation as before:
\[Eºcell=\frac{(-45 KJ/mole)(1000J/KJ)}{(1 mole)(96,485 C/mole)}=0.466 V\]
Question 12.1.2
Ozone decomposes to oxygen according to the equation 2O3(g)⟶3O2(g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen
Answer 12.1.2
- \[Rate=-\frac{Δ[O_{3}]}{2Δt}=\frac{Δ[O_{2k}]}{3Δt}\]
- When writing the rate for the reactants and products, remember that the reactants are disappearing and therefore their rate expression will be negative.
Question 12.4.20
For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene (CH2=CH–CH=CH2) has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
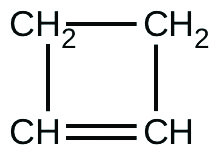
The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0 × 10−4 s−1 at 150 °C in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 °C with an initial pressure of 55 torr.
Answer 12.4.20
- Because this is a first-order reaction, we know that the equation we will use is:
\[ln(\frac{[A_{t}]}{[A_{0}]})=-kt\]
or rather
\[[A_{t}]=[A_{0}]e^{-kt}\]
- We are not given the initial concentration but we are given the initial pressure of the system, so using the first-order reaction equation we will substitute the pressure for the concentration to find the partial pressure of cyclobutene at t=30 mins:
\[[A_{30}]=[55 torr]e^{-(2.0x10^{-4}\frac{1}{sec})(30 mins)(\frac{60 sec}{1 min})}\]
\[[A_{30}]=38.37 torr\]
- Now that we have the partial pressure at t=30 mins, we can solve for the moles of cyclobutene and then solve for the concentration at that time:
\[PV=nRT\]
\[(38.37 torr)(0.53 L) = (n moles)(62.364[\frac{L*torr}{mol*K}])(423 K)\]
\[n=7.71x10^{-4} mol\]
\[Molarity=\frac{mol}{L}\]
\[M=\frac{(7.71x10^{-4}mol)}{0.53 L}\]
\[M=0.00145 \frac{mol}{L}\]
Question 21.3.4
Complete each of the following equations:
- \(_{3}^{7}\textrm{Li} + _{?}^{?}\textrm{?} \rightarrow 2[_{2}^{4}\textrm{He}]\)
- \(_{6}^{14}\textrm{C} \rightarrow _{7}^{14}\textrm{N} + _{?}^{?}\textrm{?}\)
- \(_{13}^{27}\textrm{Al} + _{2}^{4}\textrm{He} \rightarrow _{?}^{?}\textrm{?} + _{0}^{1}\textrm{n}\)
- \(_{96}^{250}\textrm{Cm} \rightarrow _{?}^{?}\textrm{?}\ + _{38}^{98}\textrm{Sr} + 4[_{0}^{1}\textrm{n}]\)
Answer 21.3.4
- In order to solve for the unknown you must set the sum of the mass numbers and atomic numbers equal to eachother (respectively). From there you can solve for the unkown mass number/atomic number, which will then tell you what element is missing from the equation.
- \[_{3}^{7}\textrm{Li} + _{1}^{1}\textrm{p} \rightarrow 2[_{2}^{4}\textrm{He}]\]
- \[_{6}^{14}\textrm{C} \rightarrow _{7}^{14}\textrm{N} + _{-1}^{0}\textrm{e}^{-1}\]
- \[_{13}^{27}\textrm{Al} + _{2}^{4}\textrm{He} \rightarrow _{15}^{30}\textrm{P} + _{0}^{1}\textrm{n}\]
- \[_{96}^{250}\textrm{Cm} \rightarrow _{38}^{98}\textrm{Sr} + 4[_{0}^{1}\textrm{n}]\]
Question 21.1.1
Identify the oxidation state of the atoms in the following compounds:
- PCl3
- (CO3)2-
- H2S
- S8
- SCl2
- Na2SO3
- (SO4)2-
Answer 21.1.1
- Chlorine is more electronegative then Phosphorus so:
Cl=-1 & P=+3, as the overall charge is zero. (+3)+3(-1)=0
- Oxygen is more electronegative than Carbon so:
O=-2 & C=+4, as the overall charge is zero. (+4)+3(-2)=-2
- Sulfur is more electronegative than Hydrogen so:
H=+1 & S=-2 as the overall charge is zero. 2(+1)+(-2)=0
- This is a pure substance (just the element Sulfur) so the oxidation state is zero:
S=0
- Chlorine is more electronegative than Sulfur so:
S=+2 & Cl=-1, as the overall charge is zero. (+2)+2(-1)=0
- Sodium always has an oxidation state of +1, and oxygen is more electronegative than sulfur so:
Na=+1 & O=-2 & S=+4, as the overall charge is zero. 2(+2)+(+4)+3(-2)=0
- Oxygen is more electronegative than sulfur so:
O=-2 & S=+6 as the overall charge is (-2). (+6)+4(-2)=-2
Question 20.4.19
Carbon is used to reduce iron ore to metallic iron. The overall reaction is as follows:
\[2Fe_2O_3 \cdot xH_2O(s) + 3C(s) → 4Fe(l) + 3CO_2(g) + 2xH_2O(g)\]
Write the two half-reactions for this overall reaction.
Answer 20.4.19
NOTE: When writing the half reactions do not include the water (initially)
- Reduction (gains electrons)
\[2Fe_2O_3 → 4Fe(l)\]
Add H2O to side that needs more oxygens
\[2Fe_2O_3 → 4Fe(l) + 6H_{2}O\]
Balance out Hydrogen on the other side now, and then balance out the charges by adding electrons
\[2Fe_2O_3 + 12H^{+} + 12e^{-} → 4Fe(l) + 6H_{2})\]
- Oxidation (loses electrons): This follows the same balancing steps as before
- \[3C(s) → 3CO_2(g)\]
- Add H2O to side that needs more oxygens
- \[3C(s) + 6H_{2}O → 3CO_2(g)\]
- Balance out Hydrogen on the other side now, and then balance out the charges by adding electrons
- \[3C(s) + 6H_{2}O → 3CO_2(g) + 12H^{+} + 12e^{-}\]
- Add H2O to side that needs more oxygens
- \[3C(s) → 3CO_2(g)\]
Question 20.9.4
Two solutions, one containing Fe(NO3)2·6H2O and the other containing the same molar concentration of Fe(NO3)3·6H2O, were electrolyzed under identical conditions. Which solution produced the most metal? Justify your answer.
Answer 20.9.4
Fe(NO3)2 would produce more metal as electrolysis of this solution would go on longer tham the solution with Fe(NO3)3. This is because in Fe(NO3)2 there are 2 molecules of NO3 for ever one Fe, while in the other solution containing Fe(NO3)3 there are 3 molecules of NO3 for every on of Fe. Because there are more NO3 molecules in the Fe(NO3)3 solution, it will become saturated at a faster rate (due to its increased surface area/size) and will therefore not produce metal for as long as the solution containing Fe(NO3)2.
Question 20.8.3
Why is it important for automobile manufacturers to apply paint to the metal surface of a car? Why is this process particularly important for vehicles in northern climates, where salt is used on icy roads?
Answer 20.8.3
- It is important for automobile manufacturers to apply paint to the metal surface of a car as is acts as a barrier between the metal and the outside world (specifically against contact with oxygen and water) thus preventing the metal from corroding. This is especially important in northern climates where salt is used on the roads, as the paint protects the metal from exposure to the salt which will rapidly increase the corroding of the metal in the form of rust.
(edit: solutions/answers looking good)

