Extra Credit 16
- Page ID
- 82874
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)17.2.5 Solution
To begin this problem, you need to assign oxidation states to each species in the reaction. Oxidation states are a formal charge assigned to elements and species in an equation for bookkeeping. To do this you need to refer to the list of rules of assigning oxidation states (OS) which are:
- Any element has an OS of zero
- The OS of a monatomic ion equals its charge
- Group 1A: +1
- Group 2A: +2
- Hydrogen: +1 with non metals, and -1 with metals and boron
- Oxygen: -1 with peroxides, and -2 with other compounds except F
- Group 7A: -1
- Fluorine: -1
- The sum of the OS's of a compound is 0
- Sum of OS's = charge of the poly atomic ion
Now that you can assign oxidation numbers, the next step is to see which element lost electrons and which elements gained electrons. An element is oxidized if it loses electrons and it is reduced if it gains electrons. You can remember this by OIL RIG (Oxidation is loss, Reduction is gain). As for the oxidizing agent and reducing agent, the oxidizing agent is the species being reduced because it is oxidizing the other species by taking an electron away from it. On the other hand, the reducing agent is the species being oxidized because it is reducing the other species by giving an electron to it.
a) \[Al(s)+Zr^{4+}(aq)\rightarrow Al^{3+}(aq)+Zr(s)\]
Oxidation States: Al=0 (Rule 1). Zr4+=+4 (Rule 2). Al3+=+3 (Rule 2). Zr=0 (Rule 1).
From this we see that the element Al goes from an OS of 0 to +3 which means that it lost 3 electrons, and the element Zr goes from an OS of +4 to 0 which means that it gained 4 electrons. We can conclude that Al(s) is the species being oxidized and Zr4+ is the species being reduced along with the fact that Al(s) is the reducing agent, and Zr4+ is the oxidizing agent from the above explanation.
b) \[Ag^{+}(aq)+NO(g)\rightarrow Ag(s)+NO^{3−}(aq)\]
Oxidation States: Ag+=+1 (Rule 2). NO(g): N=+2 and O=-2 (Rules 6 and 9). Ag=0 (Rule 1). NO-3(aq): N=+5 and 3O=-2 (Rules 6 and 9).
Answer: N increases OS by 3 which means it lost 3 electrons and the species NO(g) is the species being oxidized because it is the reactant that includes N. Ag decreases OS by 1 which means it gained 1 electron and the species Ag+(aq) is being reduced. With this information we can conclude that NO(g) is the reducing agent and Ag+(aq) is the oxidizing agent.
c) \[SiO_{3}^{2−}(aq)+Mg(s)\rightarrow Si(s)+Mg(OH)_{2}(s)\]
Oxidation States: SiO3(2-)(aq): Si=+4 and 3O=-2 (Rule 6 and 9). Mg=0 (Rule 1). Si=0 (Rule 1). Mg(OH)2: Mg=+2 and 2O=-2 and 2H=+1 (Rule 5,6 and 9).
Answer: Species being oxidized and reducing agent: Mg(s). Species being reduced and oxidizing agent: SiO3(2-)(aq).
d) \[ClO_{3}^{−}(aq)+MnO_{2}(s)\rightarrow Cl^{−}(aq)+MnO_{4}^{−}(aq)\]
Oxidation States: ClO3−: Cl=+5 and 3O=-2 (Rule 6 and 9). MnO2: Mn=+4 and 2O=-2 (Rule 6 and 9). Cl−=-1 (Rule 2). MnO4(−): Mn=+7 and 4O=-2 (Rule 6 and 9).
Answer: Species being oxidized and reducing agent: MnO2(s). Species being reduced and oxidizing agent: ClO3(−)(aq).
19.1.14 Solution
To begin this problem we need to know what the equation of percent massof an element is: Percent mass= (mass of element)/(total mass of compound) x 100.
The question gives us the mass of the element which is 2.5624-g and it also gives us the total mass of the compound which is 3.03707-g. All we have to do next is plug it into the equation and solve it.
Percent Mass= (2.5624-g)/(3.03707-g) x 100= 84.37% Chloride Ion
In order to figure out the identity of this salt we need to write the reaction equation of the chloride metal and the silver nitrate using the solubility rules to determine what the products are. Cl is (aq) because it was dissolved in water first.
\[Cl(aq)+AgNO_{3}(aq)\rightarrow AgCl(s)+NO_{3}^{-}\]
The solubility rule for this equation is that most chloride, bromide, and iodide salts are soluble except those containing Ag+, Pb(2+), and Hg+. In this case we have AgCl which is insoluble according to this rule meaning that it will form a precipitate. Now that we know this is the salt that is produced, we can identify it and this salt is Silver Chloride.
19.3.6 Solution
To determine how many unpaired electrons are present in a certain complex, we need to know how many electrons are present in the d-orbital of the metal and if the complex is high/low spin. From here we can then draw the field splitting diagram and determine how many unpaired electrons there are. The spin is already given so all we need to figure out is the number of d-orbital electrons that the metal has.
a) \[[CoF_{6}]^{3−} =high spin\]
First we look at the charge of the ligand that is attached to the metal and assign it an oxidation state (OS). The OS of F is -1 because it is in group 17, you can also figure this out with the rules of assigning oxidation states. Because there are 6 fluorine ions, we have a charge of -6 and the whole complex is -3 so Co only needs an OS of +3 to make this possible. For example, x+(-6)=-3 therefore, x=+3. From here we now look at the electron configuration of Co3+.
\[Co: [Ar]4s^{2} 3d^{7}\]
\[Co^{3+}: [Ar]3d^{6}\]
With transition metals we take the electrons away from the s-orbitals first due to the fact that they are in a higher energy orbital and are more easily removed than those electrons of the d-orbitals. From this we can see that Co3+ has 6 d-orbital electrons which will be used in creating the field splitting diagram.
The next thing you need to know is the difference between high spin and low spin. An octahedral arrangement of 6 negative charges around the metal ion causes the field splitting to have 2 orbitals raise to a higher energy level than the other three. This looks like:
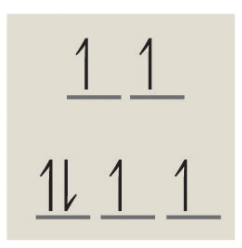
A low spin complex fills the bottom three orbitals up with 2 electrons each before moving to the upper level orbitals, and high spin complexes fill the bottom three up with 1 electron before filling the top two with a single electron. You then move back to the bottom level with the remaining electrons as needed. Since this complex is a high spin complex, we will fill each orbital up with one electron before pairing them together. The diagram would then look like:

From here we can just count the number of unpaired electrons which would be 4. Note: Since this complex has unpaired electrons it is paramagnetic.
b) \[[Mn(CN)_{6}]^{3−} =low spin\]
Following the steps in part a, the charge of the ligand CN is -1, so the charge of Mn would be +3. x+(-6)=-3 so x=+3.
\[Mn: [Ar]4s^{2} 3d^{5}\]
\[Mn^{3+}: [Ar]3d^{4}\]
4 d-electrons of low spin gives a diagram of:
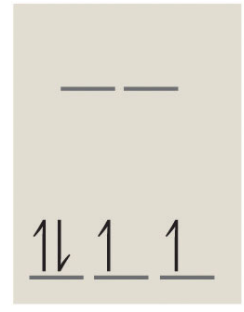
and the number of unpaired electrons is 2. Note: Since this complex has unpaired electrons it is paramagnetic.
c) \[[Mn(CN)_{6}]^{4−} =low spin\]
Charge of ligand CN: -1
Charge of metal: +2 x+(-6)=-4 x=+2
\[Mn: [Ar]4s^{2} 3d^{5}\]
\[Mn^{2+}: [Ar]3d^{5}\]
5 d-electrons of low spin gives a diagram of:
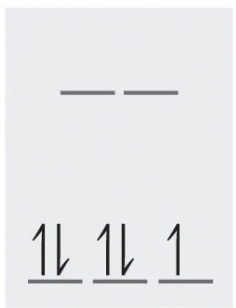
and the number of unpaired electrons is 1. Note: Since this complex has unpaired electrons it is paramagnetic.
d) \[[MnCl_{6}]^{4−} =high spin\]
Charge of ligand Cl: -1
Charge of metal: +2 x+(-6)=-4 x=+2
\[Mn: [Ar]4s^{2} 3d^{5}\]
\[Mn^{2+}: [Ar]3d^{5}\]
5 d-electrons of high spin gives a diagram of:
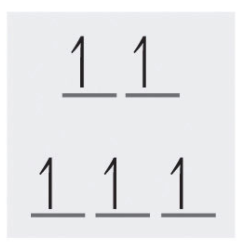
and the number of unpaired electrons is 5. Note: Since this complex has unpaired electrons it is paramagnetic.
e) \[[RhCl_{6}]^{3−} =low spin\]
Charge of ligand Cl: -1
Charge of metal: +3 x+(-6)=-3 x=+3
\[Rh: [Kr]5s^{2} 4d^{7}\]
\[Rh^{3+}: [Kr]4d^{6}\]
6 d-electrons of low spin gives a diagram of:
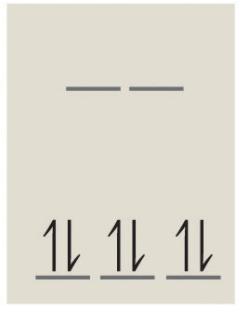
and the number of unpaired electrons is 0. Note: Since this complex has no unpaired electrons it is diamagnetic.
12.4.6 Solution
To start this problem, we need to know the equation that gives the half-life of a first order reaction. This equation is: Half-life=.693/k k=the rate constant
As you can see this reaction is independent of the final and initial concentrations so these values are not needed. Its not the reaction thats independent of concentrations its the half life. The rate constant is given to you in the question so all you need to do is plug it in and solve. Since it does not specify which units to have the answer in, just use the units given in the question.
Half-life= .693/(4.85 × 10-2 day-1) = 14.29 days
21.2.1 Solution
To write isotopes in their hyphenated form, all you need to do is state the name of the element followed by a hyphen and the mass number. The mass number is the sum of protons and neutrons in that specific atom. We only use the mass number when talking about isotopes because this is the only difference between the different isotopes since the number of protons and electrons are always the same. I would probably just stick with saying the number of protons for an element is always the same no matter the mass number since some isotopes can have a charge due to lost or gain electrons.
a) \[^{24}_{11} Na:\]
since 11 is the atomic number, the hyphenated form would use the name of the element and the number 24. Answer: Sodium-24
b) \[^{29}_{13} Al:\]
Atomic number=13; Mass number=29; Hyphenated form= Aluminum-29
c) \[^{73}_{36} Kr:\]
Atomic number=36; Mass number=73; Hyphenated form= Krypton-73
d) \[^{194}_{77} Ir:\]
Atomic number=77; Mass number=194; Hyphenated form= Iridium-194
21.5.4 Solution
Chain reactions involve fission reactions which produce more chain reactions through the products that are created. A fission reaction is the event where a larger nucleus breaks apart into smaller nuclei, and the produced nuclei are often more stable with some exceptions. One of the conditions of a nuclear chain reaction is fission for a large quantity of an element. The best example of a way that nuclear chain reactions can be controlled to produce energy but not an explosion are nuclear plants. This is because controlled chain reactions occur in nuclear reactors with many different components. These components are:
1) Fuel Element: Usually uranium or plutonium which undergo fission to provide nuclear energy.
2) Moderator: Causes neutrons to slow down in order to achieve thermal energy range.
3) Coolant: Reduces the heat produced by the fission reaction by absorbing it.
4) Control Rods: Reduces the amount of neutrons available to continue the chain reaction by absorbing them.
If all of these factors are running smoothly and there are no errors in the system, the nuclear chain reaction can be controlled to produce energy. If an error does occur, this causes accidental destructive chain reactions which results in an explosion when too much energy is produced.
20.4.2 Solution
The measured potential of an electrochemical cell depends on the Gibbs Free Energy change, and the Gibbs Free Energy change depends on many factors such as concentration and temperature. The equation for the voltage of a cell is: Eºcell=-deltaG/nF where n= number of electron moles transferred and F= Faraday constant
The equation for deltaG is: deltaG= deltaGº-RTlnQ This shows that deltaG depends on temperature and concentration which is incorporated in the Q value.
If you raise the temperature of a system, the deltaG will decrease which causes the voltage to increase. On the other hand, if the temperature is decreased, the deltaG will increase which would cause the voltage to decrease.
If you raise the concentration of the products in a system, this will increase the value of Q which would decrease deltaG causing the voltage to increase. On the other hand, if the concentration of the products are decreased, the value of Q would decrease causing deltaG to increase which in turn causes the voltage to increase.
Elias Solution. E=E°-(RT/nF)logQ If you raise the temperature you decrease the potential of the cell as you can see by the negative sign in front of the temperature containing term. If you increase the concentration of products, you will increase Q but you will be decreasing the potential due to the negative sign in front of the Q containing term. This can be coupled with Le Chateliers principle. If you had products the reverse reaction will be pushed to reduce the stress on the system from the added product and since the reverse reacting is being pushed E for the forward reaction will decrease.
20.5.31 Solution
To start this problem you need to know the relationship between Ksp and Ecell. This relationship can be described as: Eºcell= (0.0256/n)lnKsp
You then need to know the half reactions of the electrochemical cell along with their Ecell values which can be found on the Standard Reduction Potential Table.
This cell notation looks like: Ag(s)|AgBr(s)|Br-(aq)||Ag+(aq)|Ag(s) and the half reactions involved are:
\[AgBr+e_{-}\rightarrow Ag+Br{-}\]
Eºcell= +0.07133 V
\[Ag\rightarrow Ag+e^{-}\]
Eºcell= -0.7996 V (Note that the standard REDUCTION potential is .07996)
The overall Eºcell is the sum of the half reactions which is: 0.07133+(-0.7996)= -0.72827 V
If you rearrange the above equation you get: Ksp=e^(Eºcell*n/.0256) n= the number of moles of electrons transferred between the reactions which is 1 in this case.
The last step is to plug in the overall Eºcell and n and solve.
Ksp=e^(-0.7996*1/.0256)= 4.42x10^-13

