Extra Credit 13
- Page ID
- 82871
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.2
Given the following cell notations, determine the species oxidized, species reduced, and the oxidizing agent and reducing agent, without writing the balanced reactions.
Mg(s)│Mg2+(aq)║Cu2+(aq)│Cu(s)
Ni(s)│Ni2+(aq)║Ag+(aq)│Ag(s)
Answer:
Mg (s)= oxidized ; Cu2+(aq) = reduced ; Mg(s)= reducing agent ; Cu2+(aq)= oxidizing agent
Ni(s)= oxidized ; Ag+(aq) = reduced ; Ni(s) = reducing agent ; Ag+(aq) = oxidizing agent
You know Mg is oxidized because oxidation is a loss of electrons, which means a gain in positive charge. Mg loses 2 electrons to become Mg2+.The species that is oxidized is a reducing agent because it donates electrons in the redox reaction. Reduction is a gain of electrons Cu2+ gains 2 electrons to become Cu. Cu is the oxidizing agent because it acts as an electron acceptor which allows it to be reduced.
You know Ni is oxidized because oxidation is a loss of electrons and, which means a change to a more positive charge. Ni becomes Ni2+. Ni is a reducing agent because it allows reduction to occur by donating an electron. Ag+ is reduced because it gains an electron and therefore gains a negative charge to become Ag. Ag+ is the oxidizing agent because it accepts the electron that is donated by Ni.
Q19.1.11
Iron(II) can be oxidized to iron(III) by dichromate ion, which is reduced to chromium(III) in acid solution. A 2.5000-g sample of iron ore is dissolved and the iron converted into iron(II). Exactly 19.17 mL of 0.0100 M Na2Cr2O7 is required in the titration. What percentage of the ore sample was iron?
Answer:
In order to find the percentage of the ore sample that was iron we must find the weight in grams of iron used, which we have the ability to find since we titrated it against a known concentration of 0.0100 M Na2Cr2O7.
First you must write a balanced reaction based on the information given in the problem.
6Fe2+(aq) + Cr2O72-(aq) + 14H3O(aq) ⟶ 6Fe3+(aq) + 2Cr3+(aq) +21H2O(l)
We are working with a 2.5 g sample of iron and 19.7 ml of Na2Cr2O7 that is 0.01 M.
Since we are titrating the ore against the 0.01 M Cr2O72- we can use the molarity and volume to find moles of Cr2O72- used. By multiplying 0.01 M and 19.17 ml, which is 0.01917 L, we find that 1.97x10-4 moles of Cr2O72- were used.
Based on the stochiometry of the problem we know for every one mole of Cr2O72- used 6 moles of iron are needed. We can multiply 1.97x10-4 moles by 6 to find the number of moles of iron used, which is 1.1502x10-3 moles.
We know a very basic conversion to go from moles to grams. We multiply the moles of iron by the molar mass of iron, 1.1502x10-3 moles x 55.487g = .06424 g of iron.
Finally to find the percentage of iron used we simply divide the mass used, 0.06424 by the total mass 2.5 to get .02569 g. We multiply this by 100 to get a percentage of 2.57%.
Q19.3.1
Give the oxidation state of the metal, number of d electrons, and the number of unpaired electrons predicted for [Co(NH3)6]Cl3.
Answer:
Oxidation state: Co3+
We can determine the oxidation state of the metal by first looking at the charge of the ligand (NH3) which in this case is neutral. We then look at the oxidation state of the balancing anion Cl-. Our overall complex is not charged therefore our metal needs to balance out the -3 charge coming from the 3 Cl-.
Number of d electrons: 6
We determine the number of electrons by looking at the charge on Co3+ we know the first 2 electrons are lost by the S block because of their lower bond energy and the last one is lost in the d block resulting in 6 electrons.
Number of unpaired electrons: 0
Since this is an octahedral transition metal complex we have to take into account the fact that there are two spin options for this complex; high or low. We know this complex is low spin because NH3 is a strong field ligand which creates a high delta O and allows for electron pairing on the T2G level since the energy pairing here is lower than the energy required to move the electron to the Eg block. The oxidation state 3+ also affects the spin of the complex. The higher the oxidation state the stronger the ligand field.
Q12.4.3
Use the data provided in a graphical method to determine the order and rate constant of the following reaction:
2P⟶Q+W
| Time (s) | 9.0 | 13.0 | 18.0 | 22.0 | 25.0 |
|---|---|---|---|---|---|
| [P] (M) | 1.077 × 10−3 | 1.068 × 10−3 | 1.055 × 10−3 | 1.046 × 10−3 | 1.039 × 10−3 |
Answer:
Because the question asked for a graphical analysis I used an excel spread sheet to analyze the data. I found the slope of each line and determined that the reaction is second order.

Q12.7.6
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)
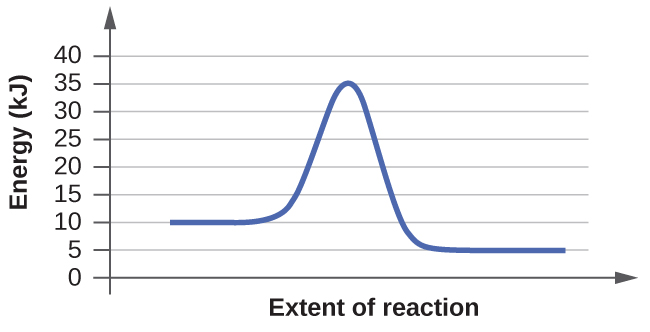
energy required- initial energy= Ea
35-10 = 25 kJ
(b)
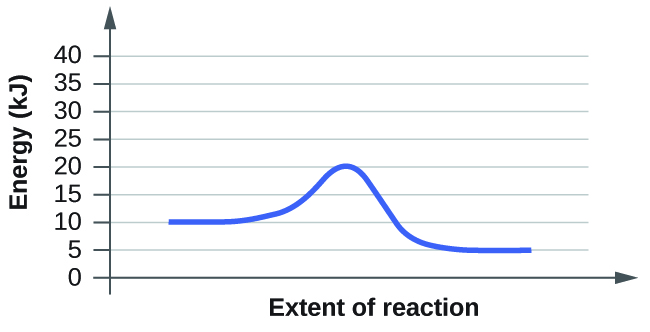
energy required- initial energy= Ea
20-10=10 kJ
Q21.5.1
Write the balanced nuclear equation for the production of the following transuranium elements:
- berkelium-244, made by the reaction of Am-241 and He-4
- fermium-254, made by the reaction of Pu-239 with a large number of neutrons
- lawrencium-257, made by the reaction of Cf-250 and B-11
- dubnium-260, made by the reaction of Cf-249 and N-15
Answer:
- 24195Am + 42He ⟶ 24497Bk + 10n
- 23994Pu + 15 10n ⟶ 254100Fm + 60-1e
- 25098Cf + 115B ⟶ 257103Lr + 410n
- 24998Cf + 157N ⟶ 260105Db + 410n
Edit: When balancing the equation one must ensure the sum of mass numbers and atomic numbers (respectively) on either side of the reaction must be equal to each other.
Q20.3.15
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
- 2Cl−(aq) + Br2(l) → Cl2(g) + 2Br−(aq)
- 2NO2(g) + 2OH−(aq) → NO2−(aq) + NO3−(aq) + H2O(l)
- 2H2O(l) + 2Cl-(aq) → H2(g) + Cl2(g) + 2OH−(aq)
- C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g)
Answer:
1. oxidation/ anode : 2Cl-(aq) → Cl2(g) + 2e- 1.35872
reduction/ cathode: 2e- + Br2(l)→ 2Br-(aq) 1.0873
Eocell= cathode- anode
Eocell= 1.0873-1.35827
Eocell=-.27097
A negative Eocell indicates a non-spontaneous reaction. The spontaneous reaction would be the reverse. The spontaneous cell diagram would be written as
Pt(s)| Br-(aq)|Br2(l)||Cl2(g)|Cl-(aq)|Pt(s)
2. oxidation/ anode: 2OH-(aq) + No2(g)→ NO3-(aq) + H2O(l) + e- -.8
reduction/ cathode: e- + NO2(g)→ NO2(aq) 1.07
Eocell= cathode- anode
Eocell= 1.07 - (-.8)= 1.87
A positive Eocell indicates a spontaneous reaction.
Pt(s)| NO2(g)| NO-2(aq)|| NO2(g)|NO-3(aq)|Pt(s)
3. oxidation/ anode: 2Cl-(aq) → Cl2(g) + 2e- -1.358
reduction/ cathode: 2e-+ 2H2O(l)→H2(g) + 2OH-(aq) -.83
Eocell= cathode- anode
Edit: Eocell= Eocathode - Eoanode
Eocell= -.83 - 1.38= -2.188
A negative Eocell indicates a nonspontaneous reaction. The spontaneous reaction would be the reverse. The spontaneous cell diagram would be written as
Pt(s)| H2O(l) |H2(g) || Cl-(aq) | Cl(g) | Pt(s)
4. oxidation/ anode: 6H2O(l) + C3H8(g) +5O2(g) → 3CO2(g) + 20H+(aq)+ 20e-
reduction/ cathode: 20e-+ 20H+(aq)+5O2(g)→ 10H2O(l)
This combustion reaction for propane is not very commonly defined in terms of an oxidation reduction reaction, however it is obviously occurring here. I could not find standard reduction potentials for these reactions so I found the Gibbs energy of formations for each element and added them up to determine ΔGo.
ΔGo=∑ΔG of products−∑ΔG of reactants
[4(-228.61) + 3(-394.39)] - [ (23.4)+ (0)] = -2361.01
This reaction has a very negative ΔGo so it is spontaneous.
Pt(s)|C3H8(g) , CO2(g)|| 5O2(g), 4H2O(g)| Pt(s)
Q20.5.28
Complexing agents can bind to metals and result in the net stabilization of the complexed species. What is the net thermodynamic stabilization energy that results from using CN- as a complexing agent for Mn3+/Mn2+?
Mn3+(aq) + e- → Mn2+(aq) E° = 1.51 V
Mn(CN)63- (aq) + e− → Mn(CN)64-(aq) E° = −0.24 V
Answer
The reaction of Mn3+ to Mn2+ is spontaneous, which we see from the positive Ecell value. CN- was used to stabilize the species so that it would become non-spontaneous, which we see through the negative Ecell value. To find the net thermodynamic stablilization energy we need to find the energy difference between each reaction and then relate this to ΔGo.
1.51 - (-0.24) = 1.75 V
ΔGo=−nFEo
ΔGo= -1(96,485)(1.75)
ΔGo= -168849 kJ

