Extra Credit 45
- Page ID
- 82805
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.4
Suppose you have three different metals, A, B, and C. When metals A and B come into contact, B corrodes and A does not corrode. When metals A and C come into contact, A corrodes and C does not corrode. Based on this information, which metal corrodes and which metal does not corrode when B and C come into contact?
S17.6.4
Here, we must be aware of definitions such as oxidation (the loss of electrons) and reduction (the gain of electrons). One substance cannot be oxidized without the reduction of another in the same chemical reaction. The term corrosion implies the unwanted oxidation of a substance and often converts a metal to a more chemically stable form. In real life, we can relate the corrosion of a substance to the rusting of an old car.
For this example, we need to keep track of which oxidizes first in each reaction. When A and B come into contact, B corrodes first and becomes the sacrificial anode, or in other terms reacts more readily than A. We can use the same method for the reaction with A and C. This time, A corrodes more readily then C, so A is more reactive than C. Since B is more reactive than A and A is more reactive than C, we can conclude that B will corrode first when B and C come into contact. This is because B is more reactive and had high ability to oxidize than C.
Original Solution is correct.
Q12.3.8
The rate constant for the radioactive decay of 14C is 1.21 × 10−4 year−1. The products of the decay are nitrogen atoms and electrons (beta particles):
What is the instantaneous rate of production of N atoms in a sample with a carbon-14 content of 6.5 × 10−9 M?
S12.3.8
The rate constant for the radioactive decay of 14C is 1.21 × 10−4 year−1. The products of the decay are nitrogen atoms and electrons (beta particles):
What is the instantaneous rate of production of N atoms in a sample with a carbon-14 content of 6.5 × 10−9 M?
Given: rate constant k= 1.21 x 10-4 year-1
= 6.5 x 10-9 M
Find: Instantaneous rate of the production of N atoms
The instantaneous rate is the reaction rate at any given point in time. Therefore, we must be able to find the reaction rate of the production of N atoms. Finding the reaction rate is as follows:
Since the reaction rate is the change in concentration of either the reactant or products over time we can define the reaction rate as,
1.1
Here, the reaction rate of N is positive because it is the production of N atoms, whereas the reaction rate of carbon-14 is negative because we are losing reactants as the reaction progresses.
From the given rate law, we can conclude that the reaction is in first order and the differential rate of of a first order reaction is,
1.2
Given 1.1, we can conclude that
1.3
Now solve for
, by using the given rate constant and the carbon-14 content.
We do this by multiplying the two numbers together,
(6.5 X 10-9M)(1.21 X 10-4 year-1) = 7.87 x 10-13 (mol/L)/year.
Original Solution is correct.
Q12.5.17
Use the PhET Reactions & Rates interactive simulation to simulate a system. On the “Single collision” tab of the simulation applet, enable the “Energy view” by clicking the “+” icon. Select the first reaction (A is yellow, B is purple, and C is navy blue). Using the “angled shot” option, try launching the A atom with varying angles, but with more Total energy than the transition state. What happens when the A atom hits the BC molecule from different directions? Why?
S12.5.17
For this problem we need to some basic knowledge on collision theory which describes the processes of rates in a chemical reaction. First, the reactants must collide with each other. Second, we need to introduce the idea of activation energy or the minimum amount of energy needed for a reaction to happen. Lastly, the reactants may have enough kinetic energy to surpass the activation energy, but fail to react because the shape or orientation of the molecules does not allow the collision to be successful.
The idea of activation energy is displayed below.
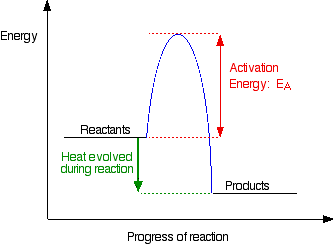
In general, if the collision is not successful, the molecules will bounce apart.
For this example, the total energy is the combined energy of the energy needed to pass the activation energy and the energy that is evolved (given off) or consumed by the reaction.
Atom A does not react with the BC molecule in any direction because the molecules did not collide in the right orientation. Therefore, the bonds are not broken even if the molecules have sufficient energy to surpass the activation energy.
Original Solution is correct.
Q21.4.12
Write a nuclear reaction for each step in the formation of from
which proceeds by a series of decay reactions involving the step-wise emission of α, α, α, α, β, β, α particles, in that order.
S21.4.12
Write a nuclear reaction for each step in the formation of from
which proceeds by a series of decay reactions involving the step-wise emission of α, α, α, α, β, β, α particles, in that order.
First, we need to classify the terms of atomic number and mass number. The atomic number is shown as a subscript on the left hand side of the atom. The atomic number tells us the number of protons and electrons in the atom. In contrast, the mass number is a combination of both the number of protons and neutrons in that atom. This number is shown as a superscript on the left hand side of the atom.
Since we are asked to write a series of step- wise emission reactions, it is important to know the product of each of emission.
The emission of an alpha particle includes the following:
Decreases the mass number(neutrons + protons) by 4, and the atomic number (number of protons) by 2
The emission of a beta particle includes the following:
Decreases the neutron number by 1, and increases the proton number by 1
The mass number does not change.
Another concept that is critical to this problem is the law of conservation of mass. From this law, the mass number and the atomic mass must be equal to each other on both sides of the equation.
We must start with . An alpha emission will result in a decrease in atomic number of 2 and a decrease in mass number of 4. We use the same process for all alpha emissions. We use the periodic table to determine the new atoms that result (looking at the atomic mass).
step 1 (alpha emission):
step 2 (alpha emission):
step 3 (alpha emission):
step 4 (alpha emission):
step 5 (beta emission):
step 6 (beta emission):
step 7 (alpha emission):
Original Solution is correct.
Q20.2.16
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
- Zn(s) + HCl(aq) →
- 3HNO3(aq) + AlCl3(aq) →
- K2CrO4(aq) + Ba(NO3)2(aq) →
- Zn(s) + Ni2+(aq) →
S20.2.16
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
- Zn(s) + HCl(aq) → H2(g) + ZnCl2(s)
- 3HNO3(aq) + AlCl3(aq) → 3HCl(aq) + Al(NO)3(aq)
- K2CrO4(aq) + Ba(NO3)2(aq) → BaCrO4(s) + 2K(NO3)(aq)
- Zn(s) + Ni2+(aq) → Zn2+(aq) + Ni(s)
1) This would be a precipitation reaction because this single replacement reaction (where one reactant takes the place of another) results in the formation of ZnCl2. We can look at the solubility table and see that Cl- will be soluble when combined with Ag+, Hg22+, and Pb2+. When a substance is soluble, that means it will not form a precipitate. Because zinc is not part of this list, then we know that ZnCl2 will precipitate.
2) This is not a redox reaction because we see no change in oxidation numbers. To figure out the oxidation numbers we look at the oxidation rules and see that the oxidation number of O is -2, H is +1, Cl is -1, and Al is +3. This is true for both sides of the equations so there is no transfer of electrons. In addition, this is not an acid-base reaction because although HNO3 is a strong acid, AlCl3 cannot act as a base. This reaction is also not a precipitation reaction because HCl and Al(NO)3 are both soluble under solubility rules.
3) This reaction qualifies as a precipitation reaction because chromates (CrO4-) are insoluble. Therefore, the product of BaCrO4 precipitates.
4) For this reaction, Zn(s) is oxidized (loss of electrons) because it has an oxidation number that goes from 0 to 2+. In contrast, Ni2+ gets reduced (gain of electrons) because its oxidation number goes from 2+ to 0. Because there is a transfer of electrons in this reaction, this would qualify as a redox reaction. The way we balance redox reactions can also include splitting the reaction into two half reactions.
Oxidation:
Reduction:
When we add these to equations, we get the following reaction shown above. Both the charge and the number of atoms should be balanced if done correctly.
Original Solution is correct.
Q20.5.11
The chemical equation for the combustion of butane is as follows:
C4H10(g)+ 13/2O2(g)→4CO2(g)+5H2O(g)
This reaction has ΔH° = −2877 kJ/mol. Calculate E°cell and then determine ΔG°. Is this a spontaneous process? What is the change in entropy that accompanies this process at 298 K?
S20.5.11
Given: ΔH° = −2877 kJ/mol
Find: E°cell , ΔG°, and the change in entropy
When both the reactants and the product are in their standard states (at one atm, 25.0°C), we can assume that K, the equilibrium constant, equals 1. Then we look at the big triangle of chemistry to determine which equation we need to use to find ΔG°.
From the information given, we must use the equation
Where R is the gas constant (8.314 J/mol) and T is the temperature at 298 K. Since K=1, then ln(1)=0 and we can conclude that = 0. As a result, the system must be at equilibrium and the reaction is neither spontaneous or non spontaneous. Remember that we can only this relationship when both the products and reactants are under standard conditions.
To calculate E°cell, we use the equation
We can describe this equation as the amount of free energy made by multiplying the cell potential with the number of transferred electrons (n). F stands for Faraday's constant (96,486 C/mol e-). Since ΔG° = 0, we can then conclude that E°cell= 0 as well.
To calculate entropy, we must you use the equation
and solve for delta S.
0= -2877kJ/mol - (298)
= -9.65 kJ/mol
Original Solution is correct.
Q24.6.7
For each complex, predict its structure, whether it is high spin or low spin, and the number of unpaired electrons present.
- [TiCl6]3−
- [CoCl4]2−
S24.6.7
1) There are six ligands in the coordination complex, indicating that is it most likely to be octahedral. Ligands have one or more donor atoms which can bind to the transition metal (in this case, Ti) and are called Lewis Bases because the donate lone electron pairs.
To figure out whether is it high or low spin, we need to look at the spectrochemical series shown below
I-< Br-< SCN-< Cl-< F- < OH- < ox < H2O < NCS-< py, NH3 < en < bpy
weak field ligands, high spin complexes strong field ligands, low-spin complexes
We should also discuss the importance of crystal field splitting between the lower and upper energy levels which is abbreviated by for octahedral substances.
Ligands with a big can bind strongly to the transition metal and are called "strong field ligands". Conversely "weak field ligands" are ligands with a small
.
In general, weak field ligands produce high-spin complexes and strong field ligands produce low-spring complexes.
For [TiCl6]3-, we see that Cl- is a weak field ligand, which then induces a high-spin complex.
In addition, Ti has a +3 charge becuase Cl has a -1 charge. The addition of these charges must equal -3 shown below.
3 + 6(-1) = -3
Looking at the periodic table, we see that Ti has an atomic mass of 22, but since it is Ti3+ the atom loses three valence electrons from its outermost shell. This means that the 4s2 electrons will come off first before taking one last valence electron to have the electron configuration of [Ar]3d1.
If we fill in the orbitals in a high spin complex, we see that their is one lone electron in the first orbital.
2) Since there are four ligands attached to the transition metal, we can infer that the complex is tetrahedral.
From the spectrochemical series, we see that Cl- is a weak ligand, so it produces a high spin complex.
In addition, Co would have a +2 charge because Cl a -1 charge. The addition of these charges must equal the charge of the complex (-2)
2 + 4(-1) = -2
Looking at the periodic table, we see that Co has an atomic mass of 27, but since it is Co2+ the atom loses 2 valence electrons from its outermost shell. This means that the 4s2 electrons will come off first and Co2+ will have an electron configuration of [Ar]3d7.
When filling in the electron orbital, we must place the electrons in a high spin complex. This means that we fill all five electrons first instead of filling one orbital with two electrons. Therefore, we get two paired orbitals on the bottom and three unpaired electrons.
Original Solution is correct.

