Extra Credit 43
- Page ID
- 82803
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.6.2
Aluminum (EºAl3+/Al=−2.07V) is more easily oxidized than iron (EºFe3+/Fe=−0.477V), and yet when both are exposed to the environment, untreated aluminum has very good corrosion resistance while the corrosion resistance of untreated iron is poor. Explain this observation.
S17.6.2
The corrosion of iron is given by:
\[ Fe^{2+} (aq) + 2OH^{-} (aq) \longrightarrow Fe(OH)_2 (s) \]
\[ 4Fe(OH)_2 (s) + O_2 (g) + 2H_2O (l) \longrightarrow 4Fe(OH)_3 (s) \]
The corrosion of aluminum is given by:
\[2Al_{(s)} +3H_2O_{(l)} \rightarrow Al_2O_{3(s)} +6H^+ +6e^-\]
When aluminum corrodes, it forms a layer of aluminum oxide [Al2O3(s)] on its surface, which blocks moisture and air from contacting the metal surface and thus no further reaction occurs. On the other hand, the iron oxides formed by corrosion of iron does not block off air and moisture, so the reaction continues until all is oxidized.
Q12.3.6
Regular flights of supersonic aircraft in the stratosphere are of concern because such aircraft produce nitric oxide, NO, as a byproduct in the exhaust of their engines. Nitric oxide reacts with ozone, and it has been suggested that this could contribute to depletion of the ozone layer. The reaction NO+O3⟶NO2+O2 is first order with respect to both NO and O3with a rate constant of 2.20 × 107 L/mol/s. What is the instantaneous rate of disappearance of NO when [NO] = 3.3 × 10−6 M and [O3] = 5.9 × 10−7 M?
S12.3.6
Since we know both NO and O3 are first order, we can write the rate of the reaction as:
\[r=k[NO][O_3]\]
given that rate constant is 2.20 x 10-7 L/mol/s, we can write:
\[r=\left(2.2*10^{-7}L/mol/s\right)[NO][O_3]\]
now plug in [NO]=3.3 x 10-6 M and [O3] = 5.9 × 10−7 M to solve for r:
\[r=\left(2.2*10^{-7}L/mol/s\right)[3.3*10^{-6} M][5.9*10^{-7}M]\]
\[r=4.3*10^{-5} M/s\]
Q12.5.15
The hydrolysis of the sugar sucrose to the sugars glucose and fructose,
\[C_{12}H_{22}O_{11}+H_2O\longrightarrow C_6H_{12}O_6+C_6H_{12}O_6\]
follows a first-order rate equation for the disappearance of sucrose: Rate = k[C12H22O11] (The products of the reaction, glucose and fructose, have the same molecular formulas but differ in the arrangement of the atoms in their molecules.)
- In neutral solution, k = 2.1 × 10−11 s−1 at 27 °C and 8.5 × 10−11 s−1 at 37 °C. Determine the activation energy, the frequency factor, and the rate constant for this equation at 47 °C (assuming the kinetics remain consistent with the Arrhenius equation at this temperature).
- When a solution of sucrose with an initial concentration of 0.150 M reaches equilibrium, the concentration of sucrose is 1.65 × 10−7 M. How long will it take the solution to reach equilibrium at 27 °C in the absence of a catalyst? Because the concentration of sucrose at equilibrium is so low, assume that the reaction is irreversible.
- Why does assuming that the reaction is irreversible simplify the calculation in part (b)?
S12.5.15
1. To find the Activation Energy, take the Arrhenius equation,
\[ E_a = \dfrac{R \ln \dfrac{k_2}{k_1}}{\dfrac{1}{T_1}-\dfrac{1}{T_2}}\]
where R=8.314 J/mol*K
k1=2.1 × 10−11 s-1
T1=27ºC+273=300K
k2=8.5 × 10−11 s−1
T2=37ºC+273=310K
\[E_a = \dfrac{\left( 8.314 J/mol*K\right) \ln \dfrac{8.5*10^{-11}s^{-1}}{2.1*10^{-11}s^{-1}}}{\dfrac{1}{300K}-\dfrac{1}{310K}}\]
\[E_a=108103.6 J*\dfrac{1kJ}{1000J}=108.1kJ\]
To find the frequency factor (Arrhenius constant), take the Arrhenius Equation and plug in Ea, k1, and T1
\[k=Ae^{\frac{-E_a}{RT}}\]
\[2.1*10^{-11}s^{-1}=Ae^{\dfrac{-108103.6J}{\left(8.314J/mol*K \right)\left(300K \right)}}\]
\[A=1.398*10^8 s^{-1}\]
To find the rate constant, plug Ea, A, and T=47ºC+273=320K into the Arrhenius Equation
\[k=\left( 1.398*10^8s^{-1}\right)e^{\dfrac{-108103.6J}{\left(8.314J/mol*K \right)\left(320K \right)}}=3.2*10^{-10}s^{-1}\]
2.Since the reaction is first order, we can use the First-Order Integrated Rate Law
\[[A]=[A]_oe^{-kt}\]
solve for t
\[\frac{[A]}{[A]_o}=e^{-kt}\]
\[ln\frac{[A]}{[A]_o}=-ktln(e)\]
\[\frac{ln\frac{[A]}{[A]_o}}{-k}=t\]
where [A]=1.65 x 10-7M , [A]o=0.15M, and k=2.1 x 10-11 s-1
\[\dfrac{ln\dfrac{[1.65*10^-7M]}{[0.15M]}}{-2.1*10^{-11}s^{-1}}=t\]
\[t=6.53*10^{11}sec(\frac{1hour}{3600sec})=1.81*10^8hours(\frac{1day}{24hours})=7.6*10^6days\]
3. So we do not have to account for any reactant that, having been converted to product, returns to the original state.
Q21.4.10
Predict by what mode(s) of spontaneous radioactive decay each of the following unstable isotopes might proceed:
- \[^{6}_2He\]
- \[^{60}_{30}Zn\]
- \[^{235}_{91}Pa\]
- \[^{241}_{94}Np\]
- 18F
- 129Ba
- 237Pu
S21.4.10
Use the Belt of Stability
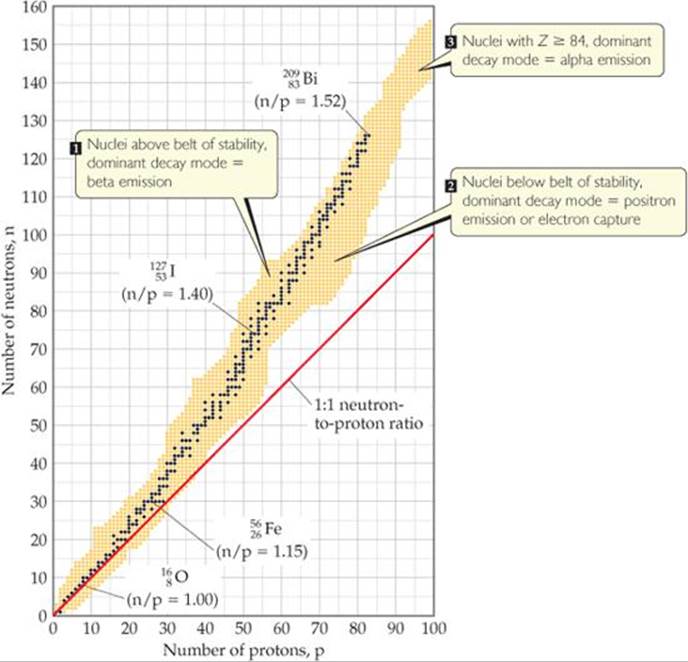
1. neutrons=6-2=4 protons=2. Helium is in the 1:1 n/p range. Too many neutrons, β decay
2. n:p ratio low, positron emission or electron capture
3. Atomic number(Z) greater than 82, α decay
4. Z>82, n=241-94=147, p=94, n>>p, α or β decay
5.n:p ratio low, positron emission or electron capture
6.n:p ratio low, positron emission or electron capture
7.n:p ratio low, positron emission or electron capture
Q20.2.14
Copper metal readily dissolves in dilute aqueous nitric acid to form blue Cu2+(aq) and nitric oxide gas.
- What has been oxidized? What has been reduced?
- Balance the chemical equation.
S20.2.14
1. Write out the (unbalanced) formula:
\[Cu(s)+HNO_3(aq)\longrightarrow Cu^{2+}(aq)+NO_2(g)\]
Write out half-reactions:
\[(ox)Cu(s)\longrightarrow Cu^{2-}(aq) +2e^- \]
\[(red)NO_3^-(aq)+e^-\longrightarrow NO_2(g)\]
Cu(s) is being oxidized, HNO3(aq) is being reduced
2. Balance the oxygen in reduction half-reaction by adding H2O. Then balance out the hydrogen with H+
\[(red) 2H^+(aq)+NO_3^-(aq)+e^-\longrightarrow NO_2(g)+H_2O(l)\]
balance the number of electrons between reduction and oxidation half-reactions:
\[Cu(s)\longrightarrow Cu^{2-}(aq) +2e^- \]
\[2*(2H^+(aq)+NO_3^-(aq)+e^-\longrightarrow NO_2(g)+H_2O(l))\]
\[4H^+(aq)+2NO_3^-(aq)+2e^-\longrightarrow 2NO_2(g)+2H_2O(l))\]
now that electrons cancel out, combine the half reactions
\[4H^+(aq)+Cu(s)+2NO_3^-(aq) \longrightarrow Cu^{2+}(aq)+2NO_2(g)+2H_2O(l)\]
Q20.5.9
Concentration cells contain the same species in solution in two different compartments. Explain what produces a voltage in a concentration cell. When does V = 0 in such a cell?
S20.5.9
Since both half-cells are composed of the same species in different concentrations, the reaction will proceed to dilute the compartment with higher molarity(M) and concentrate the compartment with lower molarity(M). Thus, voltage is produced by electrons transferring from the lower concentrated half-cell to the one higher concentration concentrated half-cell.
V=0 when the two half-cells have the same concentration, where there will be no flow of electrons. This is due to them being the same substance with the same concentration. Neither of them is more likely to reduce/oxidize.
Here is an example of a Cu(s)/Cu2+(aq) concentration cell:
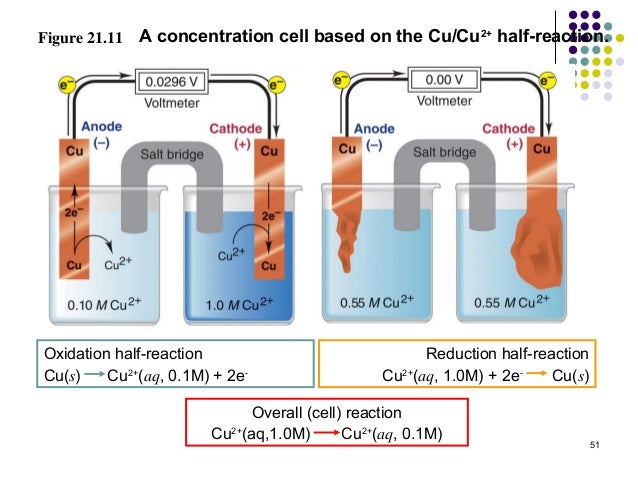
We can see that the Voltmeter reads 0.00V (right side) for the concentration cell made with same concentration of Cu2+(aq) on both sides. Whereas on the left side, V=0.0296 since there is a difference between the concentrations and thus flow of electrons.
Q24.6.5
How can CFT explain the color of a transition-metal complex?
S24.6.5
The Crystal Field Theory states that when ligands attach to a transition metal to form a complex, electrons in the d orbital split into high energy and low energy orbitals. The difference in the orbitals is splitting energy or \(∆_o\).
If we know the splitting energy, we can use the equation
\[{∆_o}=\frac{hc}{λ} \]
\[h=6.626*10^{-34}m^2kg/s\]
\[c=2.998*10^8m/s\]
to solve for λ, the wavelength of light absorbed. The complementary color(opposite on the color wheel) of that wavelength will be the color of the transition-metal complex.


