Extra Credit 32
- Page ID
- 82791
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.4.5
Use the data in Table P1 to determine the equilibrium constant for the following reactions. Assume 298.15 K if no temperature is given.
- \(\ce{AgCl}(s)⇌\ce{Ag+}(aq)+\ce{Cl-}(aq)\)
- \(\ce{CdS}(s)⇌\ce{Cd^2+}(aq)+\ce{S^2-}(aq) \hspace{40px} \textrm{at 377 K}\)
- \(\ce{Hg^2+}(aq)+\ce{4Br-}(aq)⇌\ce{[HgBr4]^2-}(aq)\)
- \(\ce{H2O}(l)⇌\ce{H+}(aq)+\ce{OH-}(aq) \hspace{40px} \textrm{at 25 °C}\)
Answer 17.4.5
(a)
Step 1: Split the reaction into half reactions.
Cathode: AgCl (s) + e- ⇌ Ag (s) + Cl- Eo= 0.2223 V
Anode: Ag (s) ⇌ Ag+ (aq) + e- Eo= 0.7996 V
Note: The cathode is where reduction (gaining an e-) occurs. The anode is where oxidation (losing an e-) occurs. Also, note that we can get the cell potential from the list of Standard Reduction Potentials.
Step 2: Use the formula Eo cell = (Eocell cathode) - (Eocell anode)
Eo cell = (0.2223 V) - (0.7996 V) = −0.5773 V
Step 3: Then use the equation: Eocell= (0.0592 V / n)(log K)
Plug in the appropriate numbers and solve for K.
-0.5773= (0.0592 / 1)(log K) note: n is one because in the reaction indicates one mol of e-
(-0.5773/0.0592)= (log K)
10^(-0.5773/0.0592)= 10^(log K)
K= 10^(-0.5773/0.0592)
K=1.77 X 10-10
* Use the same process for part B, C, and D.
(b)
Cathode: S (s) + 2e- ⇌ S2- (aq) E˚ = -0.407 V
Anode: Cd (s) ⇌ Cd2+(aq) + 2e- E˚ = -0.4030 V
Eocell= (-0.407 V) - (-0.4030 V )= -0.004 V
E˚cell= (RT/nF)lnK
Note: We have to use the equation E˚cell= (RT/nF)lnK and solve for K because the reaction is at another temperature other than standard.
Manipulate the values found above to solve for K:
K= e [(2)(96485)(-0.004)] / [(8.314)(377)]
K= = 0.782
(c)
Cathode: Hg2+(aq) + 2e- ⇌ Hg(l) E˚ = 0.7973 V
Anode: Hg (l) + 4Br- (aq) ⇌ [HgBr4]2- (aq) + 2e- E˚ =-0.4030
Eocell= (0.7973 V) - (-0.4030 V) = 1.2003 V
Manipulate to solve for K.
Eocell = (0.0592 V/ n)(log K) (Note: we can use this short cut equation because it is at standard temp.)
1.2003 = (0.0592/2)(log K)
K = 10 [(2)(1.2003)] / (0.0592)
K=3.55 x 1040 ; if you used the other eq, you would get 3.99E40 instead
(d) The equilibrium constant is just 1 x 10-14 because it is just water (remember from Chem 2b in the acids and bases unit).
Q12.1.5
A study of the rate of the reaction represented as 2A⟶B gave the following data:
| Time (s) | 0.0 | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 35.0 |
|---|---|---|---|---|---|---|---|
| [A] (M) | 1.00 | 0.952 | 0.625 | 0.465 | 0.370 | 0.308 | 0.230 |
- Determine the average rate of disappearance of A between 0.0 s and 10.0 s, and between 10.0 s and 20.0 s.
- Estimate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus [A]. What are the units of this rate?
- Use the rates found in parts (a) and (b) to determine the average rate of formation of B between 0.00 s and 10.0 s, and the instantaneous rate of formation of B at 15.0 s.
Answer 12.1.5
1. Step 1: find the average rate between 0.0 s and 10.0 s.
Average rate= (delta)[A]/(delta)(time)
Average rate= (change in concentration)/(change in time)
Between 0.0 s and 10.0 s
average rate= [([A] at 0 s) - ([A] at 10 s)] / [(0-10)]
average rate= -(1-0.625)/(0-10)= -0.0374 M/s
Step 2: Use the same method as Step 1 in order to calculate the average rate.
Between 10.0 s and 20.0 s
Average rate= (delta)[A]/(delta)(time)
Average rate= (change in concentration)/(change in time)
average rate= [([A] at 10 s) - ([A] at 20 s)] / [(10-20)]
average rate= (0.625-0.370) / (10-20)= -0.0255 M/s
2. Graph the given values in order to solve. (corrected in phase 2)
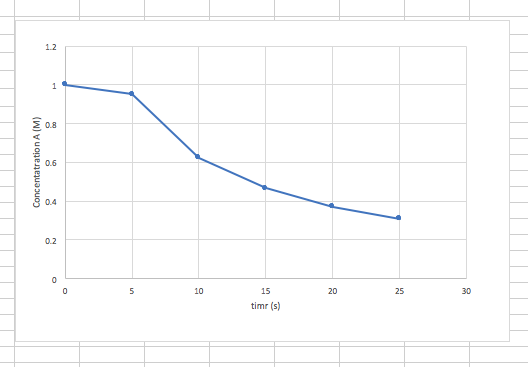
- From looking at the graph, we can estimate the instantaneous rate of disappearance of A at 15.0 s to be -0.5 M/s
3. Equation used: - (delta)[A] / (2(delta)time) = (delta)[B] / ((delta)time)
According to this relationship we determined from looking at the given reaction, we can conclude that half of the reaction rate for A is equal to the reaction time for B. The rate of reaction of A is negative while the rate of reaction of B is positive because A is the reactants and is being used up while B is the products.
-(1/2)(-0.0327 M/s) = rate B
Rate B= 0.0187 M/s
We can do the same for the instantaneous rate: (corrected in phase 2)
-(-0.05 M/s) / 2= 0.025 M/s.
Therefore, the average rate for B is 0.0187 M/s and the instantaneous rate is 0.025 M/s.
Q12.5.3
What is the activation energy of a reaction, and how is this energy related to the activated complex of the reaction?
Answer 12.5.3
Activation energy, by definition, is the amount of energy required to undergo a reaction; its the amount of energy need for the reaction to "go". This energy is related to the activated complex of the reaction because the activated complex (the complex at the top of the "bump" in the graph) requires this energy to be formed.
Another way to look at this is to look at the following diagram:

As you can see, activation energy is the difference between the energy of activated complex and energy of the reactants. Yet another way to look at it is: the energy of the reactants + activation energy = energy of the activated complex.
Q21.3.7
The mass of the atom \(_{9}^{19}\textrm{F}\) is 18.99840 amu
- Calculate its binding energy per atom in millions of electron volts.
- Calculate its binding energy per nucleon.
Answer 21.3.7
What we know:
Mass of \(_{9}^{19}\textrm{F}\) = 18.99840 amu
(delta)mass = nucleons - mass of nucleus
mass of proton=1.007276 amu
mass of neutron= 1.008664 amu
(DeltaE)=mc^2
Step 1: Find the change in mass
(delta)mass = mass of free nucleus - mass of nucleus
(delta)mass= 18.9984 - [(9) 1.0078 + (10) 1.008664]= 0.15844 amu
Step 2: Convert amu to kg
1 amu = 1.6605 x 10-27
(0.15844 amu)(1.6605 x 10-27 kg/1 amu)= 2.631E-28 kg
Step 3: Plug into the equation of E = mc2
E = mc2
E = (2.631E-28 kg)(3.00 x 108)2 = 2.3678E-11 J
Step 4: Convert to MeV
1 MeV=1.6021766E-13 J
(2.3678E-11 J)(1 MeV/1.602 x 10-13 J)= 148.8 MeV
(Note: If you round some of your numbers, you will get a slightly different answer).
To get it into MeV/nucleon:
(143.4 MeV/atom)(atom/19 nucleons) = 7.55 MeV/nucleon
(Note: we divided by 19 nucleons because that is how many nucleons there were in the atom.)
Q20.2.3
In each redox reaction, determine which species is oxidized and which is reduced:
- Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
- Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l)
- BrO3−(aq) + 2MnO2(s) + H2O(l) → Br−(aq) + 2MnO4−(aq) + 2H+(aq)
Answer 21.3.7
Strategy: Determine the oxidation states of each element on both sides of each equation. Then, figure out if the element is losing or gaining electrons. Remember the mnemonic OIL RIG.
1. Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Oxidation States:
Zn(s): 0 (oxidation states of solids are 0)
H in H2SO4(aq): +1 (SO4 has a -2 charge and that makes H a +1 charge to make the overall charge 0)
Zn in ZnSO4(aq): +2 (SO4 has a -2 charge and in order to have an overall charge of 0, Zn must be +2)
H in H2(g): 0 (diatomic molecules have an oxidation state of 0)
- The oxidation state of hydrogen went from a +1 to 0, which means it must have gained an electron. Reduction is gaining e-, therefore, hydrogen is being reduced.
- The oxidation state of zinc went from a 0 to a +2, which means it must have lost 2 electrons. Oxidation is losing e-, therefore, zinc is being oxidized.
2. Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O
Oxidation States:
Cu(s): 0 (0 because it's a solid)
N in HNO3 : +5 1 + x + 3(-2) = 0
x - 5 = 0
x = +5, so N has an o.s. of +5
Cu in Cu(NO3)2 : 2+ (NO3- has a -1 charge (and there are 2 of them), so in order for the species to be neutral, Cu must be +2)
N in NO2: +4 (-2)(2) + x = 0
x = 4, so N has a o.s. of +4
- The oxidation state of Cu has gone from 0 to a +2. Oxidation is losing e-. Cu has lost 2 electrons so Cu is oxidized.
- The oxidation state of N has gone from +5 to +4 so we know that it has gained one electron. Reduction is gaining, therefore, N is reduced.
3. BrO3−(aq) + 2MnO2(s) + H2O(l) → Br−(aq) + 2MnO4−(aq) + 2H+(aq)
Oxidation States:
Br in BrO3−: +5 x + 3(-2) = -1
x = +5
Mn in MnO2: +4 x + 2(-2) = 0
x = +4
Br−: -1 (o.s. of charged atoms is its charge)
Mn in MnO4−: +7 x + (-2)(4) = -1
x = +7
- The oxidation state of Br goes from +5 to -1, which means it has gained electrons so its being reduced.
- The oxidation state of Mn goes from +4 to +7, which means it has lost electrons so it is being oxidized.
Q20.4.22
Calculate E°cell and ΔG° for the redox reaction represented by the cell diagram Pt(s)∣Cl2(g, 1 atm)∥ZnCl2(aq, 1 M)∣Zn(s). Will this reaction occur spontaneously?
Answer 20.9.7
Half Reactions:
Zn2+(aq) + 2e- ⇌ Zn (s) Eo cell= -0.7618 V
Cl2 (g) + 2e- ⇌ 2Cl- (aq) Eo cell= 1.396 V
We know that the half reaction containing Zn is the cathode because it is on the right side of the cell diagram.
Step 1:
Eo cell = Eo cathode - Eo anode
Eo cell= (-0.7618 V) - (1.396 V)
Eo cell= -2.158 V
Step 2: Find Delta Go
ΔG°= -nFEo cell
ΔG°= -(2)(96485)(-2.1578)= 4.16 x 105
ΔG°= 4.164 x 105 J/mol
Because ΔG° is positive (and Eo cell is negative), the reaction will not occur spontaneously. (Remember that a a negative delta Go and a positive Eo cell will make a reaction spontaneous.)
Q20.9.7
What volume of chlorine gas at standard temperature and pressure is evolved when a solution of MgCl2 is electrolyzed using a current of 12.4 A for 1.0 h?
Answer 20.9.7
Equation: n = (It)/F, where n is the mol of e-, I is the current in amps, t is the time in seconds, and F is Faraday's constant (96,485 C·mol-1)
Reaction that occurs: MgCl2 (s) + 2e- → Mg (s) + Cl2 (g)
Step 1: Calculate mol of e-
n = (It)/F
n = [(12.4 A)(36000s)] / (96485 C.mol-1)= 0.463 mol of electrons
Step 2: Convert into mol of Cl2 and use the equation PV=nRT to calculate the volume of chlorine gas.
(0.463 mol e-)(1 mol Cl2 / 2 mol e-) = 0.231 mol of Cl2
(note that the conversion factor comes from the balanced equation)
Now, find the volume of chlorine gas by manipulating the equation PV=nRT:
V= (nRT) / P
= [(0.08206 L·atm·mol–1·K–1)(273 K)(0.231 mol Cl2)] / (1 atm)
= 5.175 L
Therefore, the volume of chlorine gas is 5.177 L.
Q14.6.5
Before being sent on an assignment, an aging James Bond was sent off to a health farm where part of the program’s focus was to purge his body of radicals. Why was this goal considered important to his health?
Answer 14.6.5
This goal is considered important to his health because radicals are species that are highly reactive. Radicals have an unpaired e- so it will try to pull an e- from where ever it can. They can cause instability and damage cells in Bond's body by pulling e- from inside the body and causing unwanted reactions.

