Extra Credit 24
- Page ID
- 82782
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.3.3
Determine the overall reaction and its standard cell potential at 25 °C for this reaction. Is the reaction spontaneous at standard conditions?
\[Cu(s)\vert Cu^{2+}(aq)\vert \vert Au^{3+}(aq) \vert Au(s)\]
Answer Q17.3.3
The components on the left side of the shorthand notation refers to the reaction going on in anode, while the double lines signify the salt bridge, and the right side refers to the reaction going on in the cathode. The solid Cu(s) and Au(s) are the electrodes in the galvanic cell. The single line between each of these elements separates phases, going from solid, to liquids, gases, and finally aqueous.
Remember that the anode releases electrons and is therefore where the oxidation reaction occurs, while the cathode gains electrons and is where the reduction reaction occurs.
Since the Cu(s) and Cu2+ are present on the left side, this will be at our anode and resulting oxidation reaction.
Since the Au(s) and Au3+ are present on the right side, this will be at our cathode and resulting reduction reaction.
We will write the separate oxidation/reduction reactions out like so:
\[Cu_{(s)}\rightarrow Cu^{2+}_{(aq)}\]
\[Au^{3+}_{(aq)}\rightarrow Au_{(s)}\]
Notice how the oxidation state of copper changes from 0 to 2+ (oxidation) while the oxidation of gold changes from 3+ to 0 (reduction). Keep in mind that oxidation is losing electrons, while reduction is gaining electrons.
Now, balance the equations by adding the correct number of electrons.
\[Cu_{(s)}\rightarrow Cu^{2+}_{(aq)}+2e^-\]
\[3e^-+Au^{3+}_{(aq)}\rightarrow Au_{(s)}\]
You will also need to make sure that the amount of electrons are equal for both reactions, in order to cancel this out in the end. Do this by multiplying each equation by the lowest common multiple, in this case it is 3 and 2.
\[3(Cu_{(s)}\rightarrow Cu^{2+}_{(aq)}+2e^-)\]
\[2(3e^-+Au^{3+}_{(aq)}\rightarrow Au_{(s)})\]
Cancel out like terms, and add these two reactions together:
\[3Cu_{(s)}+2Au^{3+}_{(aq)}\rightarrow3Cu^{2+}_{(aq)}+2Au_{(s)}\]
To determine standard cell potential, we will first refer to the equation \[E^{o}_{cell}=E^{o}_{cathode}-E^{o}_{anode}\]
Using the standard reduction potential table, we find that the values for the cathode and anode are: \[E^{o}_{cell}=1.50V-0.34=1.16V\]
Since Eocell > 0, this reaction is spontaneous! When the cell potential is positive that means the reaction is spontaneous because delta G =-nFEocell and a negative delta G means the reaction is spontaneous. A positive Eocell gives a negative delta G there fore this reaction is spontaneous.
Q19.1.22
Balance the following equations by oxidation-reduction methods; note that three elements change oxidation state.
\[Co(NO_3){_2(s)}⟶Co_2O{_3(s)}+NO{_2(g)}+O{_2(g)}\]
In this reaction, N changes oxidation states from +5 to +4 (reduced), Co changes oxidation states from +2 to +3 (oxidized), and O changes oxidation states from -2 to 0 (also oxidized).
First, split this reaction into its oxidation and reduction half reactions, and balance all of the elements that are not hydrogen or oxygen (we will deal with these later):
\[Reduction: 2Co(NO_3){_2}\rightarrow Co_2O_{3}+4NO_2\]
Now, for the oxidation reaction, we are only dealing with O2 on the products side. In order to balance this, we will need to add water and hydrogen to both sides:
\[Oxidation: 2H_2O\rightarrow O_2+4H^+ \]
Balance the amount of oxygens on each side by adding the correct number of water molecules (H2O), and balance the amount of hydrogen by adding the correct number of H+ atoms:
\[Reduction: 2H^++2Co(NO_3){_2}\rightarrow Co_2O_{3}+4NO_2+H_2O\]
\[Oxidation: 2H_2O\rightarrow O_2+4H^+\]
Finally, balance the charges by adding electrons to each side of the equation. For the reduction reaction, we will add 2 electrons to balance out the 2H+, and to the oxidation reaction, we will add 4 electrons to balance out the 4H+. Remember, the goal of this step is to make sure that the charges are balanced, so we can cancel them out in the end.
\[Reduction: 2e^- + 2H^++2Co(NO_3){_2}\rightarrow Co_2O_{3}+4NO_2+H_2O\]
\[Oxidation: 2H_2O\rightarrow O_2+4H^+ +4e^-\]
Multiply the reduction reaction by two, in order to balance the charges so there are 4 electrons on each side of the reaction.
\[Reduction: 2(2e^- + 2H^++2Co(NO_3){_2}\rightarrow Co_2O_{3}+4NO_2+H_2O)\]
and combine both reactions which comes out to:
\[2H_2O + 4Co(NO_3)_2 + 4H^+ \rightarrow 2CO_2O_3 + 8NO_2 + 2H_2O + O_2 + 4H^+\]
Cancel out like terms:
\[4Co(NO_3){_2(s)} \rightarrow 2CO_2O{_3(s)} + 8NO{_2(g)} + O{_2(g)}\]
Both sides have overall charges of 0 and can be checked to see if they are balanced.
Q12.4.14
There are two molecules with the formula C3H6. Propene, CH3CH=CH2, is the monomer of the polymer polypropylene, which is used for indoor-outdoor carpets. Cyclopropane is used as an anesthetic:
When heated to 499 °C, cyclopropane rearranges (isomerizes) and forms propene with a rate constant of 5.95 × 10−4 s−1. What is the half-life of this reaction? What fraction of the cyclopropane remains after 0.75 h at 499.5 °C?
Answer Q12.4.14
Use the equation \[ t{_1}{_/}{_2} = \frac{ln2} k\] since this is a first-order reaction. You can tell that this is a first order reaction due to the units of measurement of the rate constant, which is s-1. Different orders of reactions lead to different rate constants, and a rate constant of s-1 will always be first order.
Plug into the equation, and you get half life = 1164.95 seconds. To convert this to hours, we would divide this number by 3600 seconds/hour, to get 0.324 hours.
Use the integrated first order rate law \[ln\frac{[A]}{[A]_0} = -kt\]. In this equation, [A]0 represents the initial amount of compound present at time 0, while [A] represents the amount of compound that is left after the reaction has occurred. Therefore, the fraction \[\frac{[A]}{[A]_0}\] is equal to the fraction of cyclopropane that remains after a certain amount of time, in this case, 0.75 hours.
Substitute x for the fraction of \[\frac{[A]}{[A]_0}\] into the integrated rate law: \[ln\frac{[A]}{[A]_0} = -kt\] \[ln(x) = -5.95x10^{-4}(0.75)\] \[x=e^{(-0.000595)(0.75)}\] = 0.20058 = 20%.
So, the half life is 0.324 hours, and 20% of the cyclopropane will remain as 80% will have formed propene.
Q21.2.9
Which of the following nuclei lie within the band of stability?
- chlorine-37
- calcium-40
- 204Bi
- 56Fe
- 206Pb
- 211Pb
- 222Rn
- carbon-14
Answer Q21.2.9
The band of stability represents a set of certain isotopes that are stable. This stability is determined by the ratio of protons and neutrons in the nucleus. This ratio of protons and neutrons can be determined from A (the mass number = number of neutrons + protons) and Z (the atomic number = number of protons). When Z is less than 20, it is a small number and likely not stable. But, if Z is greater than 20, a larger amount of neutrons will be needed to balance out the protons in order for it to be stable. In addition, if Z is greater than 83 it is radioactive.
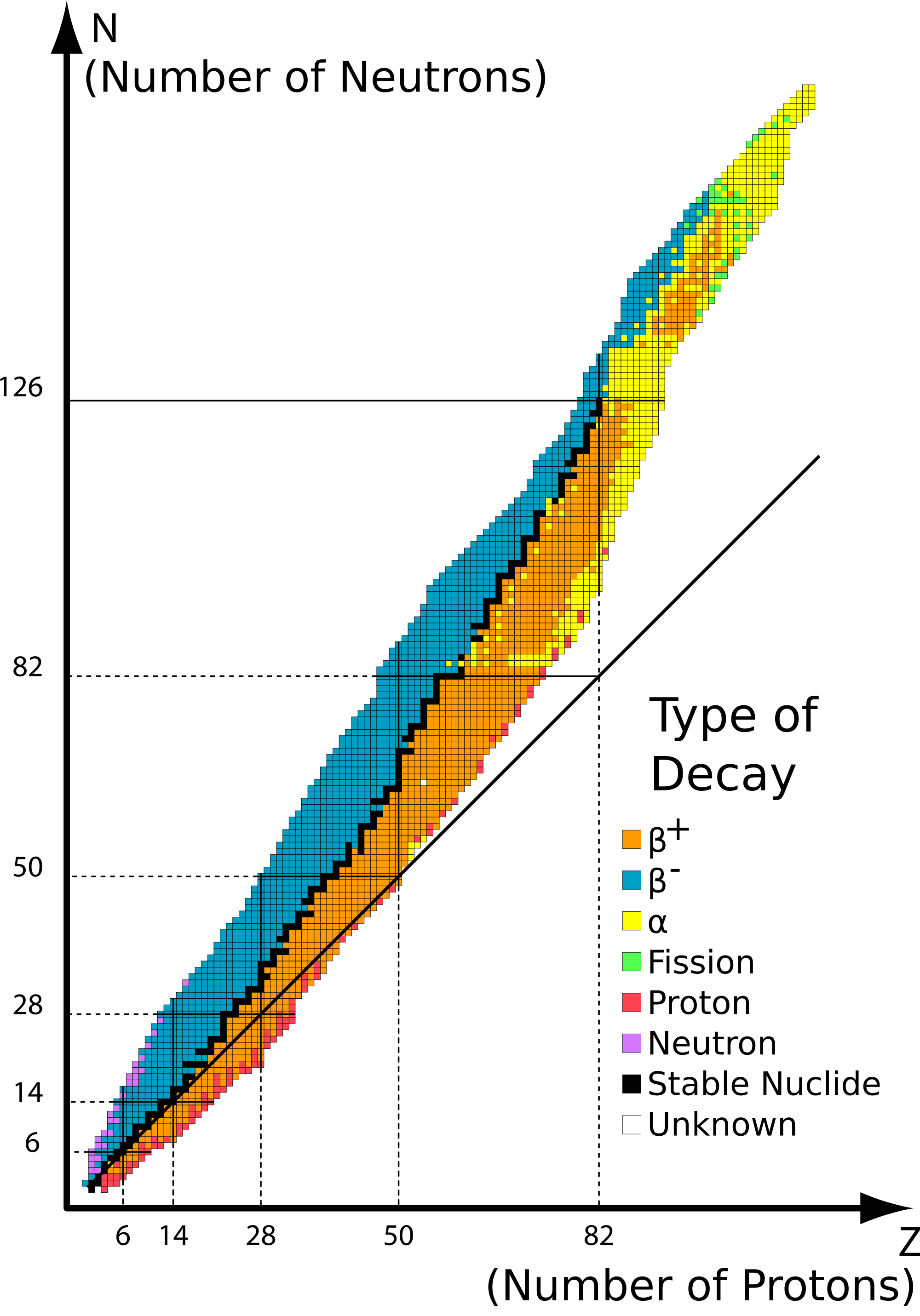
This stability can be determined by looking at certain "magic numbers", or simply graphing it on the belt of nuclear stability, and seeing if it matches up with the line of stability. If the nucleus is not on the belt of stability, it will undergo either beta emission, positron emission, or alpha decay, depending on the position on the graph.
We know that even numbers are (usually) more stable than odd. The more close in number they are, the more likely that the nuclei is stable. The best way to determine whether a nuclei is stable or not is to compare it to the band of stability, but calculating the amount of protons and neutrons are in it.
The number of protons and neutrons can be determined by this equation:
\[A=N+Z\]
Where A = mass number, N = number of neutrons, and Z = atomic number, found on the periodic table.
Chlorine-37 contains 17 protons and 20 neutrons. This contains one of the magic numbers, 20, and also lies on the belt of stability.
Calcium-40 contains 20 protons and 20 neutrons. Since these numbers are equal, they are very likely to be stable. In addition, this also lies on the belt of stability when you graph it.
204Bi contains 83 protons and 121 neutrons. Atomic numbers with more than 20 protons require more neutrons than protons to be stable. When graphing this, it also ends up to lie on the belt of stability
56Fe contains 26 protons and 30 neutrons. This answer was previously incorrect. This does lie within the band of stability, but is hard to see in the graph. Since the proton number is above 20, it requires more neutrons to be stable. Also, if the nucleon number is even meaning if both the proton and the neutron numbers are even, it has more of a chance of being stable.
206Pb contains 82 protons and 124 neutrons. When graphing this, we find that this does lie on the band of stability. 82 is also one of the proton magic numbers.
211Pb contains 82 protons and 129 neutrons. When graphing this, we find that this lies above the belt, and is not stable.
222Rn contains 86 protons and 136 neutrons. When graphing this, we find that this also lies above the belt and is not stable.
Carbon-14 contains 6 protons and 8 neutrons. When graphing this, we find that this also lies above the belt and is not stable.
Therefore, a, b, c, d, and e are the correct answers.
Q21.7.1
If a hospital were storing radioisotopes, what is the minimum containment needed to protect against:
- cobalt-60 (a strong γ emitter used for irradiation)
- molybdenum-99 (a beta emitter used to produce technetium-99 for imaging)
Answer Q21.7.1
Since cobalt-60 emits gamma rays, the hospital would need to store the radioisotopes in a substance such as lead, since this is the only material that gamma rays will stop at. We need a material that the rays would not be able to pass through, which, in this case would be lead.
Similarly, since molybdenum-99 emits beta rays, the hospital would need to store the radioisotopes in some type of metal, for example, aluminum. This is a material that the beta rays would not be able to pass through, and would therefore contain the beta rays released during the radioactive decay.
The diagram explains what type of radiation can pass through which materials.

Q20.4.10
![]()
For each application, describe the reference electrode you would use and explain why. In each case, how would the measured potential compare with the corresponding E°?
- measuring the potential of a Cl−/Cl2 couple
- measuring the pH of a solution
- measuring the potential of a MnO4−/Mn2+ couple
Answer Q20.4.10
A reference electrode is an electrode with an accurately maintained potential, that is used as a reference when measuring cell potentials in galvanic cells. The most common reference electrode that we use is the standard hydrogen electrode. On the standard reduction potential chart, the standard hydrogen electrode has a value of 0, and all other electrical potentials are written down compared to this one.
From the chart, the standard hydrogen electrode has the reaction \[2H^+(aq)+2e^-\rightarrow H_2(g)\]
1. I would choose the SHE as the reference electrode. The standard hydrogen electrode (SHE) has a measured voltage of 0, which makes it easier to compare with other reduction reactions on the standard reduction potential table. The Eo value for the Cl-/Cl2 reaction is 1.358, while the potential of the SHE is 0. This would be a much stronger reduction reaction than the reference electrode, since it is at a much higher standard reduction potential value. Is SCE an option?
2. When measuring the pH of a solution, I would choose a pH electrode. This would be a glass electrode sensitive to the measurement of hydrogen ions. This is commonly used in the measurement of pH of a solution. A glass electrode is typically thought of as a tube within a tube with the inner one containing an HCl solution at a constant molarity. This basically acts as a galvanic cell, and is effective in the measurement of pH of a solution. SCE reference electrode could be used to measure pH as well.
3. For the last one, I would also use the standard hydrogen electrode (SHE) as the reference electrode because it has a measured voltage of 0. This makes it easier to compare to the other values on the table. The Eo value for this reaction is 1.51 compared to the SHE, which is much higher. This would make this reaction a better and stronger reduction reaction than the reference electrode.
The measured potential in all of these cases will still differ slightly from the actual Eo value. The measured potential depends on the equation \[E=E^o-\frac{RT}{n}lnQ\] This equation takes in to account Q, the reaction quotient, which is determined by dividing the molarities of the reactants by the molarities of the products. Because of this, the measured potential changes according to the different concentrations of the various compounds going through the oxidation/reduction reactions.
Q20.4.11
![]()
Draw the cell diagram for a galvanic cell with an SHE and a copper electrode that carries out this overall reaction:
\[H_{2(g)} + Cu^{2+}_{(aq)}\rightarrow 2H^+_{(aq)} + Cu_{(s)}\].
Answer Q20.4.11
Shorthand cell diagrams are constructed with solid electrodes on the outside, then liquid, gas, and aqueous. If there is no solid electrode present in the reaction, like there is in this equation, then you would use either Pt(s) or C(s) (graphite) as the electrode. There is a single straight line separating each of these phases. In the middle, the anode (left side) is separated from the cathode (right side) by a double bar that represents the salt bridge in between the galvanic cells.
The phases are written in parentheses, with 1 atm and 1M being used as the standard concentration for gases and aqueous/solid solutions, respectively.
\[Pt(s)\vert H_2(g,1 atm)\vert H^+(aq,1M) \vert\vert Cu^{2+}(aq) \vert Cu(s)\]
Q20.8.2
![]()
What does it mean when a metal is described as being coated with a sacrificial layer? Is this different from galvanic protection?
Answer Q20.8.2
When a metal is being coated with a sacrificial layer, this basically means that a sacrificial anode is being attached to the metal, which will oxidize first, before the metal being protected. In other words, a metal surface will be introduced to another, more electronegative and reactive metal, which will cause electrons to flow from the newly introduced metal onto the original metal. This changes the labels of both of these metals, as the new metal will be known as the anode, and the old metal is now the cathode of the reaction. Because the metal that is sacrificed is more likely to oxidize, it gets oxidized before the other metal, depending on where it is on the standard reduction potential table.
This will prevent the metal underneath from corroding first. This is also known as cathode protection, or galvanic protection.

