Extra Credit 23
- Page ID
- 82781
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Question 17.3.2
For each reaction listed, determine its standard cell potential at 25 °C and whether the reaction is spontaneous at standard conditions.
1. \(Mn(s) + Ni^{2+}(aq) ⟶Mn^{2+}(aq) + Ni(s)\)
2.\(3Cu^{2+}(aq)+2Al(s)⟶2Al^{3+} (aq)+2Cu(s)\)
3. \(Na(s)+LiNO_3(aq)⟶NaNO_3(aq)+Li(s)\)
4.\(Ca(NO_3)_2(aq)+Ba(s)⟶Ba(NO_3)_2(aq)+Ca(s)\)
Answer 17.3.2
For these types of reactions, we must separate the reactions into the oxidation reaction where the electron is present in the products side and the reduction reaction where the electron is present in products side. We can then label these as the anode or cathode where the anode is where oxidation occurs and the cathode is where the reduction occurs. We do these two types to determine the voltage of the reaction and which is the cathode and which is the anode to plug it in correctly to the equation:

Once we have identified the Cathode and the Anode, we just find their corresponding voltage of the chart and we plug in to find the cell potential. However, the second part of the question asks us to determine if the reaction is spontaneous or not. A reaction is spontaneous when Ecell>0 because the Gibbs free energy of the reaction will be negative so the reaction will be spontaneous but is Ecell<0 the reaction will not be spontaneous.
1. We can start by breaking down Mn(s)+Ni2+(aq)⟶Mn⟶Mn2+(aq)+Ni(s) .
Mn → Mn2++ 2e- this will be the oxidation side because of the loss of an electron which tells us that this is the anode and by looking at the chart we can tell that its cell potential is -1.17
Ni2++2e- → Mn this will be the reduction side because of the gain of an electron which tells us that this is the cathode and by looking at he chart we can tell that its cell potential is -.257
All we have to do now is plug into the equation to get E0cell= -.257-(-1.17)=.913v and since this is a positive value, it tells us that it is spontaneous.
2. For the second problem, we can approach it the same way, by breaking down and disregarding the coefficients because they don't matter in these types of problems 3Cu2+(aq)+2Al(s)⟶2Al3+(aq)+2Cu(s)
Cu2++2e- → Cu gain of an electron, meaning reduction, which means cathode. Voltage according to the chart is .34v.
Al → Al3++3e- loss of an electron, meaning oxidation, which means anode. Voltage according to the chart is -1.66.
We can plug this into the equation to get E0cell=.34v-(-1.66)=2.0v and since it's positive, the reaction will be spontaneous.
For the third and fourth reaction, we notice the inclusion of a spectator ion that will help us determine which is cathode and which is the anode.
3. For this problem we have : Na(s)+LiNO3(aq)⟶NaNO3(aq)+Li(s)
In this case, NO3- is the spectator ion so we can ignore it in the eq, however, we know that it has a negative charge so we are going to have a Li+ in the reactant side and Na+ in the reactant side:
Li+ + e- → Li
This will be the reduction side since we gain an electron and that also means that it is the cathode and from the chart we get -3.04v
Na → Na+ + e -
This will be the oxidation side since we lose an electron and that also means that it is the anode and from the chart we get -2.71v
Plug this into the standard E0cell equation we get: E0 cell= -3.04-(-2.71)=-.33v and since this value is negative, the reaction is not spontaneous.
4. This problem follows the same method as number 3, we have: Ca(NO3)2(aq)+Ba(s)⟶Ba(NO3)2(aq)+Ca(s)
NO3- is again the spectator ion so Ca2+ will exist on the reactant side and Ba2+ will exist on the reactant side.
Ca2+ + 2e- → Ca
Since this is a gain of an electron, it is defined as reduction which also means that it is on the Cathode side and by looking at the chart we get that voltage is -2.87.
Ba → Ba2++2e-
Since this is a loss of an electron, it is defined as oxidation which also means that is on the anode side and by looking at the chart we get that voltage is -2.90.
Plugging this into the standard equation, we get: E0 cell= -2.87-(-2.90)=.03 and since this is positive, the reaction is spontaneous.
Question 19.1.21
Predict the products of each of the following reactions and then balance the chemical equations.
- Fe is heated in an atmosphere of steam.
- NaOH is added to a solution of Fe(NO3)3.
- FeSO4 is added to an acidic solution of KMnO4.
- Fe is added to a dilute solution of H2SO4.
- A solution of Fe(NO3)2 and HNO3 is allowed to stand in air.
- FeCO3 is added to a solution of HClO4.
- Fe is heated in air.
Answer 19.1.21
Predicting the products of reactions can be very simple, you just have to know key terms.
1. For the first problem it tells us that Iron is heated in an atmosphere of steam, we can instantly point out the steam is just water in a gaseous form so we can just write out the equation where in the products, the oxygen combines with the Fe in a single replacement
\(Fe(s)+H_2O→ Fe_3O_4(s)+H_2(g)\)
Now that we have this equation we can then now proceed to balance both sides and get:
\[3Fe(s)+ 4H_2O → Fe_3O_4(s)+ 4H_2(g)\]
2. For this problem, we are told that NaOH is added to a solution of Fe(NO3)3 so we can start off by writing NaOH(aq)+Fe(NO3)3
We can tell that double replacement must occur but since it is in solution and Nitrate and Sodium become soluble so they dissociate. So we can write the equation and balance it as:
3NaOH(aq)+ Fe(NO3)3 → Fe(OH)3 (s) + 3Na+(aq) + 3NO3- (aq)
3. For this reaction we are told that FeSO4 is added to an acidic solution of KMnO4 so we can start off by writing the reactants side:
FeSO4+KMnO 4, for this reaction we can also tell that double-replacement must occur so we proceed and balance and write the equation as :
FeSO4 +2KMnO4 → Fe(MnO4)2+K2SO4
\[8H_2SO_4 (aq)+10FeSO4(aq) +2KMnO_4(aq) → 2MnSO_4 (aq) +5 Fe_2(SO_4)_3(aq) + K_2SO_4 (aq) + 8 H_2O (l) \]
4. For this reaction, we are told that Fe is added to a dilute solution of H2SO4 which tells us that water must be in the product, the only way that can occur is if SO2 is a by product and Fe is mixed with SO4 so we can write the equation as
\[2Fe + 6H_2SO_4 → Fe_2(SO_4)_3 + 3SO_2 + 6H_2O\]
\[Fe(s)+H_2SO_4 (aq) → FeSO_4 (aq) + H_2 (g) \]
5. For this reaction, we are told that a solution of Fe(NO3)2 and HNO3 are allowed to stand in air which tells us that the presence of O2 will exist on the reactant side and will result in water and the dissociation of the other ions.
\[4Fe^{2+}(aq)+O_2(g)+4HNO_3(aq)⟶4Fe3^+(aq)+2H_2O(l) + 4NO_3^-(aq)\]
6. For this reaction we are told that FeCO3 is added to a solution of HClO 4, again the key word solution tells us that we are going to have a double replacement with a liquid since H2O will be produced and a gas will be present since we will have CO2, so we can write this equation and balance it and write:
FeCO3 + 2HClO4 → Fe(ClO4)2 + H2O + CO2
7. For this reaction we are told that Fe is heated in air, from this we can tell that air represents O2 so we can add Fe and O2 and this will be a synthesis and balance it and result in:
3Fe + 2O2 → Fe3O4
Question 19.3.13
\([CuCl_4]^{2-}\) is green. \( [Cu(H_{2}O)_6]^{2+}]\) is blue. Which absorbs higher-energy photons? Which is predicted to have a larger crystal field splitting?
Answer 19.3.13
Although a color might appear a certain way, it actual absorbs a different color, opposite of it on the color wheel. 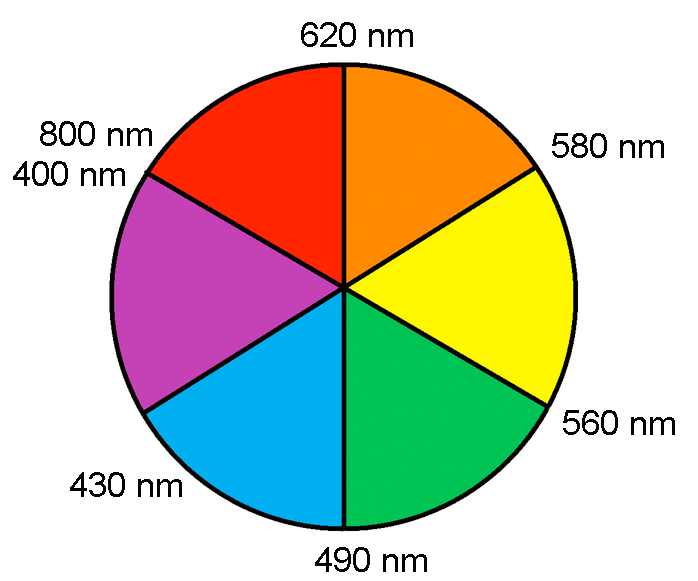
In this case;
[CuCl4]2- appears green but is opposite of red on the color wheel which is absorbed and is characterized by wavelengths 620-800 nanometers.
[Cu(H2O)6]2+ appears blue but is opposite of orange on the color wheel which is absorbed and is characterized by wavelengths 580-620 nanometers.
When determining which absorbs the higher energy photons, one must look at the complex itself. A higher energy indicates a high energy photon absorbed and a lower energy indicates a lower energy photon absorbed. How can we determine this? By looking at the complex and more specifically the ligand attached and its location in the spectrochemical series.
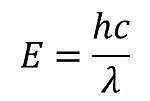

The ligands attached are Water and Chlorine and since Water is a stronger ligand than Chlorine according to the series, it also has larger energy, indicating a higher energy. This means that the complex [Cu(H2O)6]2+ absorbs a higher energy photon because of its a stronger ligand than chlorine.
Part 2 of this question also asks which complex is predicted to have a larger crystal field splitting. To determine this you also use the spectrochemical series and see which ligand is stronger. Since H2O is stronger than Cl- on the spectrochemical series, we can say [Cu(H2O)6]2+ has a higher crystal field splitting.
Question 12.4.13
Both technetium-99 and thallium-201 are used to image heart muscle in patients with suspected heart problems. The half-lives are 6 h and 73 h, respectively. What percent of the radioactivity would remain for each of the isotopes after 2 days (48 h)?
Answer 12.4.13
This problem is asking us for the percentage of radioactivity remaining after a certain time for both isotopes after 48 hours. We must identify an equation that will help us solve this and we can determine that we can determine this information using the first order equation.
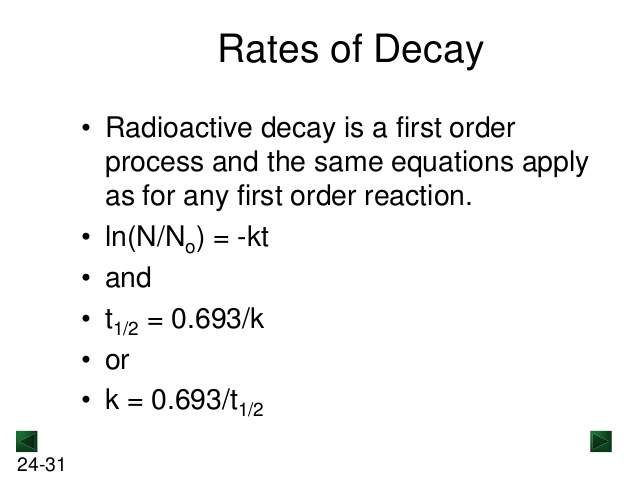
This equation Ln(N/No)= -kt tells that the Natural log of the fraction remaining is equal to the rate constant times time. To determine the rate constant, we can also compute .693 over the half-life given in the information.
For Technetium-99 we can determine the rate constant by plugging into the second equation: .693/6 hrs= .1155 h-1
Now that we have the rate constant we can plug in : Ln(N/No)=-(.1155h-1)(48h) so Ln(N/No)=-5.544 and if we take the inverse of the natural log, we get (N/No)=3.9x10 -3 and if we multiply this by 100, we get .39% remaining.
We can do this same process for Thallim-201 and plugin: .693/73 hrs= .009493151 h-1 and when we plug this into the first order equation we get:
Ln(N/No)=-(.009493151h-1)(48h) so Ln(N/No)=-.45567248 and when we take the inverse of the natural log, we get (N/No)=.6340 and when multiplied by 100, we get 63.40% remaining which makes sense since its half-life is 73 hours and only 48 hours have passed, half of the amount has yet to be consumed.
Question 21.2.8
The mass of the atom 1123Na is 22.9898 amu.
- Calculate its binding energy per atom in millions of electron volts.
- Calculate its binding energy per nucleon.
Answer 21.2.8
1. To calculate binding energy, we must first identify the equation which is Einstein's Theory of Relativity:

Now that we have identified our equation, we look more into the mass defect. We are looking for energy per atom in millions of electron volts. We have the speed of light so all we need to find is the mass defect. To do this, we find the number of protons and number of neutrons (mass number - atomic number) and we multiple them by their mass, 1.0078u and 1.0087u respectively and then subtract that number by 22.9898amu which is given to find the mass defect:
m=(11*1.0078+12*1.0087)-22.9898=0.2004u. We now have the mass defect but the question asks for binding energy per atom in millions of electron volts.
Plugging in all the values, we get:
\( E = (0.2004 amu)(1.6605*10^{-27} kg/1 amu )({3.00*10^8})^2 \)
\(E = 2.993*10^{-11} {J/ nucleus} \)
1 MeV = \( 1.602 {J/ nucleus} \)
Converyting to MeV :
\( (2.993*10^{-11} {J/ nucleus})*(1.602 {J/ nucleus} ) = 186.8 MeV / nucleus\)
2. Using the information we have learned from part one, we can try to find the binding energy per nucleon. Since we know that mass defect is .2004u, we can multiple this number by 1.6605x10-27 Kg to get 3.327642x10-28kg so that we can convert our units. Now that we have the mass defect in kg, we can have done the difficult step and we can now plug-in to the equation:
E=(3.327642x10-28kg)(2.998x10-8ms-1)2 and we get 3.00x10-11J but we are not done yet! The problem asks for binding energy per nucleon, so we must divide our answer by 23 to get the binding energy per nucleon:
3.00x10-11J/23 nucleon= 1.30x10-12J/nucleon
Question 21.6.3
Iodine that enters the body is stored in the thyroid gland from which it is released to control growth and metabolism. The thyroid can be imaged if iodine-131 is injected into the body. In larger doses, I-131 is also used as a means of treating cancer of the thyroid. I-131 has a half-life of 8.70 days and decays by β− emission.
- Write a nuclear equation for the decay.
- How long will it take for 95.0% of a dose of I-131 to decay?
Answer 21.6.3
1. To write the nuclear equation you must first know that beta decay consists of \(^0_{-1}e^- \). You must also know now that elements are written in the form \(^A_{Z}X \) , where A represent the mass number and the atomic number represents the number of protons. With this information, you can now write the decay equation with the beta particle on the products side.
13153I → 0-1e + 13154Xe
The Mass numbers and atomic numbers must add up on both sides. It is better to start off with the atomic number. Since we have 53 on the reactant side and it goes through beta decay with -1, 53=-1+x where x= 54 and the element with 54 protons is Xenon. Also, the mass number in this reaction does not change so Xenon takes 131=0+x and X is 131 so the mass number is unchanged.
2. To calculate the time for 95% to a dose to decay, we must use the first order equation again where k=.693/half life and Ln(N/No)=-kt
We must first plug in to try to find K so that we can plug it into the amount remaining equation:
k=(.693/8.70days)= .079655172 d-1 , now that we have this, we can plug in to find the time for 95% to decay, however, we must be careful and plug-in .05 because that it the fraction of dose that will remain:
Ln(.05)= -(.079655172d-1)T , taking the natural log and dividing but the rate constant, we find that T=37.6 days which tells us that it will take 37.6 days for 5% of the dose to remain or 95% to decay
Question 20.4.9
All reference electrodes must conform to certain requirements. List the requirements and explain their significance.
Answer 20.4.9
In an electrochemical cell, a reference electrode can be used as the half cell. A reference electrode is an electrode which has a stable and well-known electrode potential. The high level of stability of the electrode is reached by occupying constant concentrations of each participant in the oxidation-reduction reaction. There are several requirements in order for a reference electrode to exist. The composition must be fixed so that the accuracy and stability of the system stay intact so changes in measured potential are only due to changes in analyte concentration. Another major requirement for a reference electrode is that the potential does not change with time. Since the passage of current through an electrode can alter the potential, such effects are minimized by having a high input impedance and by using a non-polarizable electrode as the reference electrode so that the passage of small currents does not alter the potential.

