Extra Credit 22
- Page ID
- 82780
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)revisions made in red
Q17.3.1
For each reaction listed, determine its standard cell potential at 25 °C and whether the reaction is spontaneous at standard conditions.
a) \[Mg(s)+Ni^{2+}(aq)\rightarrow Mg^{2+}+Ni(s)\]
b) \[2Ag^{+}(aq) + Cu(s)\rightarrow Cu^{2+}(aq) + 2Ag(s)\]
c) \[Mn(s) + Sn(NO_{3})_{2}(aq)\rightarrow Mn(NO_{3})_{2}(aq)+Sn(s)\]
d) \[3Fe(NO_{3})_{2}(aq) + Au(NO_{3})_{3}(aq)\rightarrow 3Fe(NO_{3})_{3}(aq)+Au(s)\]
S17.3.1
When finding standard cell potential you are going to use the equation,
\[E^{o}_{cell}=E_{cathode}^{o}- E_{anode}^{o}\]
Along with a list of standard reduction potential values at 25 degrees Celsius
First step you have to do is split each equation into half reaction equations. One equation will represent the oxidation equation, and the other will represent the reduction equation. Oxidation means that the molecule is losing electrons while reduction means that the molecule is gaining electrons. So for the first one:
a) \[Mg(s)+Ni^{2+}(aq)\rightarrow Mg^{2+}+Ni(s)\]
The oxidation half-reaction equation would be:
\[Mg(s)\rightarrow Mg^{2+}+2e^{-}\]
The reduction half-reaction equation would be:
\[Ni^{2+}+2e^{-}\rightarrow Ni(s)\]
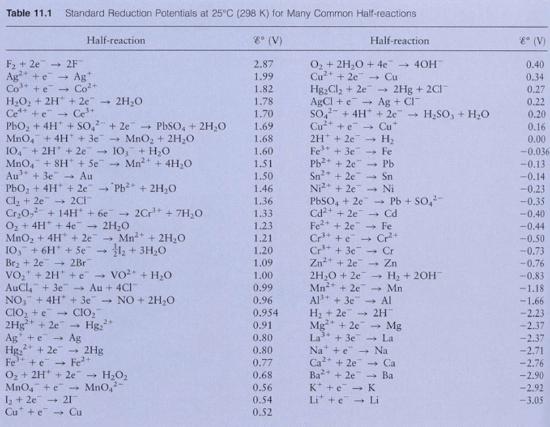
Next step is to look at the values for standard reduction potentials. You will see that for the first half reaction for Mg(s), the standard reduction value is -2.37V, and the second half reaction for Ni(s) is -.23V
Next you have to plug in the value you just looked up into the equation
\[E^{o}_{cell}=E_{cathode}^{o}- E_{anode}^{o}\]
Because oxidation occurs at the anode, you will plug in the value you received from the oxidation equation into the anode part of the standard cell equation. Then you plug the reduction value into the cathode part of the equation.
\[E^{o}cell=-.23-(-2.37)\]
\[E^{o}cell=2.14\]
Because the standard cell potential is "+", this means that the reaction is spontaneous. According to this equation,
\[\Delta G=-nFE^{o}\]
If you plug in a "+" standard cell potential value, Delta G will be negative, causing the reaction to be spontaneous. F stands for Faraday's Constant which is 96485 C mol-1, n is the number of electrons canceled from the half-reactions which in this case is 2, and Ecell is what we calculated previously to be 2.14V.
\[\Delta G=-2(96485)(2.14)\]
\[\Delta G=-412955.8\]
A negative ΔG means the reaction is spontaneous.
b) \[2Ag^{+}(aq) + Cu(s)\rightarrow Cu^{2+}(aq) + 2Ag(s)\]
Half reaction equations
Oxidation: \[Cu(s)\rightarrow Cu^{2+}(aq)+2e^{-}\]
Reduction: \[2Ag^{+}(aq)+2e^{-}\rightarrow 2Ag(s)\]
\[E^{o}Cell=.80-.34\]
\[E^{o}Cell=.46\]
Standard cell potential is "+" so the reaction is spontaneous
c) \[Mn(s) + Sn(NO_{3})_{2}(aq)\rightarrow Mn(NO_{3})_{2}(aq)+Sn(s)\]
Oxidation: \[Mn(s)\rightarrow Mn^{2+}+2e^{-}\]
Reduction: \[Sn^{2+}+2e^{-}\rightarrow Sn\]
\[E^{o}cell=-.14-(-1.18)\]
\[E^{o}cell=+1.04\]
Positive Ecell means the reaction is spontaneous.
d) \[3Fe(NO_{3})_{2}(aq) + Au(NO_{3})_{3}(aq)\rightarrow 3Fe(NO_{3})_{3}(aq)+Au(s)\]
Because NO_3 is a spectator ion, meaning it appears on both sides of the equations, you do not need to include it in the half reaction equations.
Oxidation:\[Fe^{2+}\rightarrow Fe^{3+}-e^{-}\]
Reduction:\[Au^{3+}+3e^{-}\rightarrow Au\]
\[E^{o}Cell=1.50-.77\]
\[E^{o}Cell=+.73\]
Positive Ecell means the reaction is spontaneous.
Q19.1.20
What is the gas produced when iron(II) sulfide is treated with a nonoxidizing acid?
S19.1.20
Iron(II) sulfide \[FeS(s)\]
If Iron (II) sulfide was treated with a nonoxidizing acid, an acid that cannot act as an oxidizing agent, for example, HCl, it would look like this:
\[FeS(s)+2HCl\]
Our equation would be
\[FeS(s)+2HCl\rightarrow H_{2}S(g)+FeCl_{2}(s)\]
Thus, the gas produced when iron(II) sulfide is treated with a nonoxidizing acid would be Hydrogen Sulfide.
Q19.3.12
Would you expect salts of the gold ion, Au+, to be colored? Explain.
S19.3.12
No, Au+ would not be colored because it has a full 5d sublevel. Since there are no ligands involved there is no value for delta o, therefore it can not absorb nor reflect light. According to the equation:\[E=hc/\lambda\] energy is the reflection of light and lambda is the wavelength of absorbed light. Since there is no absorbed light there will be no light reflected, making Au+ colorless.
Q12.4.12
Some bacteria are resistant to the antibiotic penicillin because they produce penicillinase, an enzyme with a molecular weight of 3 × 104 g/mol that converts penicillin into inactive molecules. Although the kinetics of enzyme-catalyzed reactions can be complex, at low concentrations this reaction can be described by a rate equation that is first order in the catalyst (penicillinase) and that also involves the concentration of penicillin. From the following data: 1.0 L of a solution containing 0.15 µg (0.15 × 10−6 g) of penicillinase, determine the order of the reaction with respect to penicillin and the value of the rate constant.

S12.4.12
First thing you have to do is determine the order of the reaction, by looking at the chart, when the concentration of Penicillin doubles, the rate doubles. This means that the reaction is first order because the rate is occurring at the same pace as the concentration. To determine the value of the rate constant "k" you have to use this equation. First order was determined by looking at the concentrations relative to the rate. When the concentrations doubled, the rate only doubled, this is a first order. If the concentration doubled and the rate quadrupled the order would be second.
\[Rate=k[X]^{m}\]
Plug into the equation one concentration for Penicillin, plug in the corresponding rate, and plug in the order number into "m" to look like:
\[(1.0x10^{-10})=k[2.0x10^{-6}]^{1}\]
isolate "k" and calculate.
\[k=((1.0x10^{-10})/(2.0x10^{-6}))\]
\[k=5x10^{-5} mol^{-1} min^{-1}\]
Q21.2.7
What are the two principal differences between nuclear reactions and ordinary chemical changes?
S21.2.7
Nuclear reactions are when two nuclei collide to form a different nucleus, therefore, elements get transmuted into other elements. Ordinary chemical reactions use the transfer of electrons in their reaction. Ordinary chemical reactions involve the transfer of, loss of, gain of, and sharing of electrons. Also, nuclear reactions involve much larger energies because the potential energy of the nucleus is MUCH larger than the potential energy of electrons. Also, since nuclear reactions have a higher energy, their mass change is more detectable as well.
Nuclear energy also deals with mass to energy conversions as seen in fission and fusion reactions, whereas ordinary chemistry deals with chemical energy being converted to another usable form. Nuclear energy is a much higher scale of energy due to the large conversion in the Einstein's equation: E=mc^2. This is the conversion of lost mass, or mass deficit, of lone particles, neutrons and protons, versus combined particles. This mass deficit is very small but when multiplied by the square of the speed of light squared it makes a large difference.
Q21.6.2
Technetium-99m has a half-life of 6.01 hours. If a patient injected with technetium-99m is safe to leave the hospital once 75% of the dose has decayed, when is the patient allowed to leave?
S21.6.2
Because half life is independent of the initial concentration, this reaction will be in the first order. For a first order half life problem you will be using:
\[t_{1/2}=[A]_{o}/2k\]
and
\[ln([A]_{t}/[A]_{o})=-kt\]
The first thing you have to do is find "k". Since you have the half life time, you will use the 1st equation to find "k".
\[6.01=ln(2)/k\]
\[k=.1153\]
plug "k" into the 2nd equation along with the concentration at a certain time, the original concentration, and "k":
\[ln([.25]/[1])=-(.1153)t\]
\[t=12.02\]
The patient is allowed to leave in 12.02 hours.
Q20.4.8
Identify the oxidants and the reductants in each redox reaction.
a) \[Br_{2}(l)+2I^{-}(aq)\rightarrow 2Br^{-}(aq)+I_{2}(s)\]
b) \[Cu^{2+}(aq)+2Ag(s)\rightarrow Cu(s)+2Ag^{+}(aq)\]
c) \[H^{+}(aq)+2MnO_{4}^{-}(aq)+5H_{2}SO_{3}(aq)\rightarrow 2Mn^{2+}(aq)+3H_{2}O(l)+5HSO_{4}^{-}(aq)\]
S20.4.8
Each equation will split up into two half reaction equations, a reduction equation and an oxidation equation. In the oxidation equation there will be a reduction agent, which oxidizes, and in the reduction equation, there will be an oxidation agent, which reduces. The coefficients are not necessary to include when splitting the original equation into half reactions
a) \[Br_{2}(l)+2I^{-}(aq)\rightarrow 2Br^{-}(aq)+I_{2}(s)\]
Oxidation: \[2I^{-}\rightarrow I_{2}+2e^{-}\]
The reducing agent is the reactant in the equation, losing the electron.
Reduction:\[Br_{2}+2e^{-}\rightarrow 2Br^{-}\]
The oxidizing agent is in the reactant, gaining the electron.
b) \[Cu^{2+}(aq)+2Ag(s)\rightarrow Cu(s)+2Ag^{+}(aq)\]
Oxidation: \[2Ag\rightarrow 2Ag^{+}+2e^{-}\]
Reducing agent:\[2Ag\]
Reduction: \[Cu^{2+}+2e^{-}\rightarrow Cu\]
Oxidizing agent: \[Cu^{2+}\]
c) \[H^{+}(aq)+2MnO_{4}^{-}(aq)+5H_{2}SO_{3}(aq)\rightarrow 2Mn^{2+}(aq)+3H_{2}O(l)+5HSO_{4}^{-}(aq)\]
Reduction: \[MnO_{4}^{-}\rightarrow Mn^{2+}\]
In order to get the full half reaction equations we have to first balance the oxygens, then the hydrogens, and then finally, the electrons, on both sides. Once both equations have a balanced Oxygens, hydrogens, and electrons, we can cancel out molecules on opposite sides of the equation for both equations to get the original equation again (this is to check our work to make sure we balanced the equations correctly).
\[5e^{-}+MnO_{4}^{-}+8H^{+}\rightarrow Mn^{2+}+4H_{2}O\]
\[10e^{-}+2MnO_{4}^{-}+16H^{+}\rightarrow 2Mn^{2+}+8H_{2}O\]
If we were to cancel out the molecules on opposite sides for both equations, then we have the correct half balanced equations.
To determine the reducing agent, we have to look at the transfer of electrons for the transition medal. In this cause, the Mn in the reactants has an oxidation number of +7 while the Mn in the products has an oxidation number of +2, this means that in the reaction, Mn gained electrons. This reaction is now the reduction equation and the oxidizing agent is 2MnO_4^-.
Oxidation: \[H_{2}SO_{3}\rightarrow HSO_{4}^{-}\]
\[H_{2}SO_{3}+H_{2}O\rightarrow HSO_{4}^{-}+3H^{+}+2e^{-}\]
\[5H_{2}SO_{3}+5H_{2}O\rightarrow 5HSO_{4}^{-}+15H^{+}+10e^{-}\]
The oxidation number for S in the reactants is +4 and the oxidation number for S in the products is +6. This means that S lost electrons, making the equation the oxidation equation and 5H_2SO_3 the reducing agent.
Q20.7.5
This reaction is characteristic of a lead storage battery:
\[Pb(s)+PbO_{2}(s)+2H_{2}SO_{4}(aq)\rightarrow 2PbSO_{4}(s)+2H_{2}O(l)\]
If you have a battery with an electrolyte that has a density of 1.15 g/cm3 and contains 30.0% sulfuric acid by mass, is the potential greater than or less than that of the standard cell?
S20.7.5
First we must calculate the moles of sulfuric acid:
We are given density: 1.15g/cm3 and that the battery contains 30.0% weight by mass. This allows us to calculate the total mass of sulfuric acid and convert sed mass into moles.
\[\frac{1.15g}{cm^{3}}\cdot \frac{.30}{1}\cdot \frac{1 mole}{98.079 grams}\cdot \frac{1,000 grams}{1 Liter}\]
The above equation converts density from grams to moles, accounts for the 30% by mass and converts grams/cm3 to grams per liter
This gives us the molarity of:
\[Molarity= 3.517 M\]
We have more moles of sulfuric acid in this battery than in standard batteries, we can see how this deviates from the standard cell potential by using the equation below:
\[E= E_{cell potential}^{o}-\frac{RT}{nF}\cdot ln(Q)\]
The larger value of sulfuric acid changes the value of Q:
\[Q=\frac{Products}{Reactants}\]
By increasing the reactants we are decreasing the overall value of Q
According to the graph of natural logarithms:
As the graph gets small and below 1 it becomes more negative!
By increasing the amount of reactants we have decreased the value of Q and thus made the number a larger negative.
If you look at the equation again:
\[E= E_{cell potential}^{o}-\frac{RT}{nF}\cdot ln(Q)\]
You and we use a larger negative for ln(Q) we will thus increase the value of Ecell potential
Thus the Ecell potential > Eocell potential