Extra Credit 14
- Page ID
- 82770
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.3
For the cell notations in the previous problem, write the corresponding balanced reactions.
a.) \(Mg(s)\mid Mg^{2+}(aq)\left | \right |Cu^{2+}(aq)\mid Cu (s)\)
The first step is to find out which part is the anode reaction and which part is the cathode reaction. The anode reaction is on the left side of the double vertical lines and the cathode reaction is on the right side.
anode: \(Mg(s)\rightarrow Mg^{2+}(aq)+2e^{-}\)
cathode: \(Cu^{2+}(aq)+2e^{-}\rightarrow Cu(s)\)
Next, we want to add up both equations for an overall reaction and cancel out the electrons.
\(Mg(s)+Cu^{2+}(aq)\rightarrow Mg^{2+}(aq)+Cu(s)\)
b.) \(Ni(s)\mid Ni^{2+}(aq)\left | \right |Ag^{+}(aq)\mid Ag (s)\)
The first step is to find out which part is the anode reaction and which part is the cathode reaction. The anode reaction is on the left side of the double vertical lines and the cathode reaction is on the right side.
anode: \(Ni(s)\rightarrow Ni^{2+}(aq)+2e^{-}\)
cathode: \(Ag^{+}(aq)+e^{-}\rightarrow Ag(s)\)
Multiple the entire cathode equation by 2 in order to cancel out the electrons.
cathode: \(2\times (Ag^{+}(aq)+e^{-}\rightarrow Ag(s))\) which gives cathode: \(2Ag^{+}(aq)+2e^{-}\rightarrow 2Ag(s)\)
Next, we want to add up both equations for an overall reaction and cancel out the electrons.
overall: \(2Ag^{+}(aq)+Ni(s)\rightarrow 2Ag(s)+Ni^{2+}(aq)\)
Q19.1.12
How many cubic feet of air at a pressure of 760 torr and 0 °C is required per ton of Fe2O3 to convert that Fe2O3 into iron in a blast furnace? For this exercise, assume air is 19% oxygen by volume.
The equation that is derived is \(Fe_{2}O_{3}+6CO_{2}\rightarrow 2Fe+3CO_{2}+3CO\) and also \(2C+O_{2}\rightarrow 2CO\)
We must find the mol of Fe2O3 per ton.
1 ton = 907.18474 kg = 907,184.74 g
907,184.74 g x (1 mol Fe2O3 / 159.69 g) = 5680.91 moles Fe2O3
Then we must convert into the number of moles of CO2 needed to react with the iron (II) oxide to form iron.
5680.91 mol Fe2O3 X (6 mole CO2 / 1 mol Fe2O3 ) = 34085.46834 moles CO2
Then, we must plug it into the gas equation for volume at stp. Convert 760 torr into 1 atm and use .08206 L*atm/ mol *K for the gas constant.
PV=nRT
(1 atm)(V)=(34085.46834 mol)(.08206 L atm/ mol K)(273.15K)
V= 764015.1723 L
Then we divide by the 19% and convert from m3 (liters) to ft3.
V= 145162.88 m3 X 35.3147 = 5,126,383.66 ft3
*originally incomplete; done by phase 2
Q19.3.4
The solid anhydrous solid CoCl2 is blue in color. Because it readily absorbs water from the air, it is used as a humidity indicator to monitor if equipment (such as a cell phone) has been exposed to excessive levels of moisture. Predict what product is formed by this reaction, and how many unpaired electrons this complex will have.
Since we know that the solid CoCl2 will absorb water from air, we get the molecule H2O to combine with the Co. The product formed by this reaction will be [Co(H2O)6]Cl2 since the H2O is neutral in this compound. To find the number of unpaired electrons this complex must have, we must look at the oxidation state of the Co. In this compound, H2O is neutral and the oxidation state of Cl2 is -2. Thus, the oxidation state of Co must be +2 in order for the compound to remain neutral. So, we would get a ion of Co2+ which has the electron configuration of 4s23d7. Since the ion has a 2+ charge, we need to take away 2 electrons from the lowest orbital, which is the 4s2.As a result, there will be 7 electrons.In an octahedral, the crystal field diagram would show 3 t2g orbitals on the bottom with 2 eg orbitals above.
Since H2O is a weak field ligand, its crystal field diagram will exhibit high spin. When adding electrons, we must follow the pauli exclusion principles and Hund's rule. Therefore arrows must be started at the lowest orbital energy and fill up one arrow before it can move to the next orbital. All five orbitals will first have only one arrow and then begin pairing when the remaining 2 electrons are distributed. Thus, there would be 3 unpaired electrons.
Q12.4.4
Pure ozone decomposes slowly to oxygen, \(2O_{3}(g)\rightarrow 3O_{2}(g)
\). Use the data provided in a graphical method and determine the order and rate constant of the reaction.
| Time (h) | 0 | 2.0 × 103 | 7.6 × 103 | 1.23 × 104 | 1.70 × 104 | 1.70 × 104 |
|---|---|---|---|---|---|---|
| [O3] (M) | 1.00 × 10−5 | 4.98 × 10−6 | 2.07 × 10−6 | 1.39 × 10−6 | 1.22 × 10−6 | 1.05 × 10−6 |
In order to determine the order of the reaction, we must make 3 graphs. One with concentration vs time which corresponds to the zero order, next with ln(concentration) vs time corresponding to first order, and the last with 1/ concentration vs time corresponding to second order. Which ever graph is linear, we can determine the order of the reaction. When graphing 1/ concentration vs time, we get this graph.
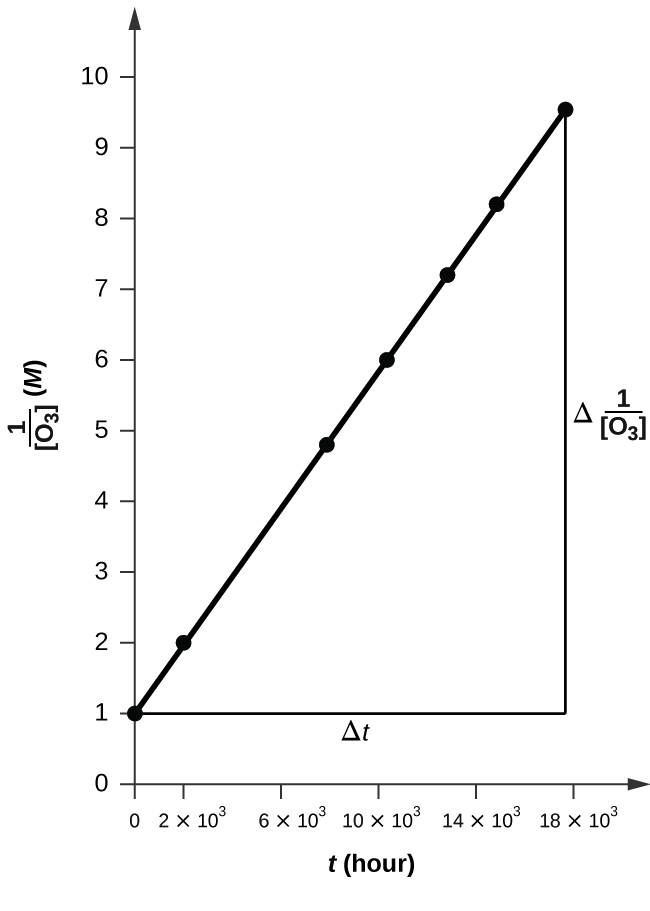
The plot is nicely linear, so the reaction is second order.
In order to find the rate of change of the concentration of O3, we must plug in data from the first two points on the graph into the equation
\(rate=\Delta[O_{3}]/2\Delta t\)
\(rate=(4.981\times 10^{-6}-1\times 10^{-5})/2(2\times 10^{3}-0)\)
\(rate=1.255\times 10^{-9}\)
Then plug this value and the [O3] from experiment 2 into the proposed rate law
rate=k[O3]2
\(1.255\times 10^{-9}=k[4.98\times 10^{-6}]^{2}\)
Now solve for K
k = 50.6 M−1 h−1
*origininally incomplete; solution to K done by phase 2
Q12.7.7
For each of the following reaction diagrams, estimate the activation energy (Ea) of the reaction:
(a)
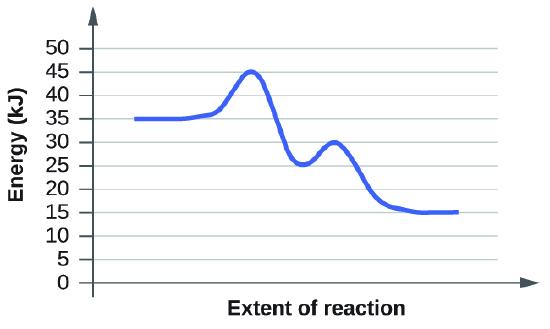
The activation energy is the minimum amount of energy needed to undergo a specific reaction. In this graph, we start off with 35 kJ as the reactants. Then we look at the first transition sate which is at 45 kj. Subtract the transition state by the energy of the reactants and you will get 10 kj.
(b)
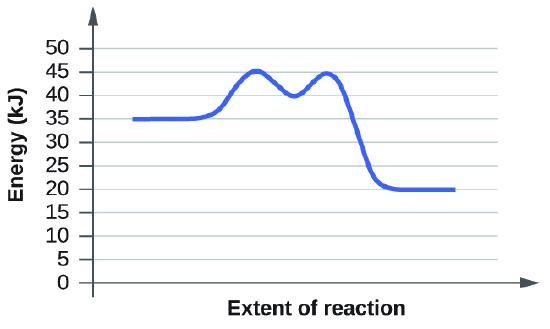
The activation energy is the minimum amount of energy needed to undergo a specific reaction. In this graph, we start off with 35 kJ as the reactants. Then we look at the firt transition sate which is at 45 kj. Subtract the transition state by the energy of the reactants and you will get 10 kj
Q21.5.2
How does nuclear fission differ from nuclear fusion? Why are both of these processes exothermic?
Nuclear fission is different from nuclear fusion because fission releases energy by splitting nuclei. Nuclear fusion releases energy by joining nuclei, which is just the opposite process. In retrospect, fission is the splitting of an atom into two or more smaller ones while fusion is the fusing of two or more smaller atoms into a larger one. Both of these processes are exothermic since fusion only works (releases energy) on light nuclei, and fission only works on heavy ones.
Q20.3.16
Write the half-reactions for each overall reaction, decide whether the reaction will occur spontaneously, and construct a cell diagram for a galvanic cell in which a spontaneous reaction will occur.
- \(Co(s)+Fe^{2+}(aq)\rightarrow Co^{2+}(aq)+Fe(s)\)
The half reactions for this equation is \(Co(s)\rightarrow Co^{2+}(aq)+2e^{-}\) which is the anode and \(Fe^{2+}(aq)+2e^{-}\rightarrow Fe(s)\) which is the cathode.
To determine spontaneity, find \(E^{\circ}_{cell}\) by subtracting the standard reduction potential of the anode from that of the cathode.
\(E^{\circ}_{cell}=E^{\circ}_{cathode}-E^{\circ}_{anode}\)
\(E^{\circ}_{cell}=-0.44-(-0.28)\)
\(=-0.16\)
The reaction is NOT spontaneous because \(E^{\circ}_{cell}> 0\) when spontaneous
In order to make the reaction spontaneous, simply switch the anode and cathode. Since the conditions weren't specifed, one could assume standard conditions and therefore the concentrations will be equal to 1 M.
\(Fe(s)\mid Fe^{2+}(aq)(1M)\parallel Co^{2+}(aq)(1M)\mid Co(s)\)
2. \(O_{2}(g)+4H^{+}(aq)+4Fe^{2+}(aq)\rightarrow 2H_{2}O(l)+4Fe^{3+}(aq)\)
The half reactions for this equation is \(Fe^{2+}\rightarrow Fe^{3+}+e^{-}\) which is the anode and \(4e^{-}+4H^{+}+O_{2}(g)\rightarrow 2H_{2}O(l)\) which is the cathode.
\(E^{\circ}_{cell}=E^{\circ}_{cathode}-E^{\circ}_{anode}\)
\(E^{\circ}_{cell}=1.229-0.771\)
\(=0.458\)
The reaction is spontaneous. Once again, assume standard conditions and make the partial pressure of the gas equal to 1 atm. Since the reaction for the anode does not include a solid reactant or product, we must introduce an inert electrode. In this example, we used Platinum as the inert electrode, however one can also use C(graphite).
\(Pt(s)\mid Fe^{3+}(aq)(1M),Fe^{2+}(aq)(1M)\parallel O_{2}(g)(1atm)\mid H_{2}O(l)\mid Pt(s)\)
3. \(6Hg^{2+}(aq)+2NO_{}3^{-}(aq)+8H^{+}\rightarrow 3Hg_{2}^{2+}(aq)+2NO(g)+4H_{2}O(l)\)
The half reactions are
Anode: \(2Hg^{2+}(aq)\rightarrow Hg_{2}^{2+}(aq)+2e^{-}\)
Cathode: \(6e^{-}+8H^{+}+2NO_{3}^{-}(aq)\rightarrow 2NO(g) + 4H_{2}O(l)\)
\(E^{\circ}_{cell}=E^{\circ}_{cathode}-E^{\circ}_{anode}\)
\(E^{\circ}_{cell}=0.96-0.92\)
\(=0.04\)
The reaction is spontaneous
\(Pt(s)\mid Hg^{2+}(aq)(1M),Hg_{2}^{2+}(aq)(1M)\parallel NO_{3}^{-}(aq)(1M)\mid NO(g)(1atm)\mid Pt(s)\)
4. \(CH_{4}(g)+2O_{2}(g)\rightarrow CO_{2}(g)+2H_{2}O(g)\)
The half reactions for this equation are
Cathode: \(8e^{-}+8H^{+}+2O_{2}(g)\rightarrow 2H_{2}O(g)+2H_{2}O(l)\)
Anode: \(2H_{2}O(l)+CH_{4}(g)\rightarrow CO_{2}(g)+4H^{+}+4e^{-}\)
Since this is a combustion reaction, it is spontaneous.
\(C(graphite)\mid CH_{4}(g),CO_{2}(g)\parallel O_{2}(g),H_{2}O(g)\mid C(graphite)\)
Q20.5.29
You have constructed a cell with zinc and lead amalgam electrodes described by the cell diagram Zn(Hg)(s)∣Zn(NO3)2(aq)∥Pb(NO3)2(aq)∣Pb(Hg)(s). If you vary the concentration of Zn(NO3)2 and measure the potential at different concentrations, you obtain the following data:
| Zn(NO3)2 (M) | Ecell (V) |
|---|---|
| 0.0005 | 0.7398 |
| 0.002 | 0.7221 |
| 0.01 | 0.7014 |
- Write the half-reactions that occur in this cell.
- The half reactions that occur in this cell are \(Zn(Hg)(s)+e^{-}\rightarrow Zn(NO_{3})(aq)\) and \(Pb(NO_{3})_{2}(aq)\rightarrow Pb(Hg)(s)+e^{-}\)
- What is the overall redox reaction?
- The overall redox reaction is Zn(Hg)(s) + Pb(NO3)2(aq)→Zn(NO3)2(aq) + Pb(Hg)(s).
- What is E°cell? What is ΔG° for the overall reaction?
- To determine the E°cell we must use the Nernst equation because the cell is not under standard conditions since it has varying concentrations of Zn(NO3)2 . We may assume it is at room temperature still, however, because we were not told otherwise.
- E°cell=.7398
- To find ΔG°, plug into The Big Triangle. ΔG°= -71380.34
- What is the equilibrium constant for this redox reaction? To find the equilibrium constant for this redox reaction, we must plug into the equation from the big triangle.
- K= 4.55x1013

