Extra Credit 12
- Page ID
- 82768
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 17.2.1
Write the following balanced reactions using cell notation:
a. \(Mg(s) + Ni^{2+}(aq)\rightarrow Mg^{2+}(aq)+Ni(s)\)
Solution: First identify the oxidation and reduction half reactions:
\[oxidation: Mg(s)\rightarrow Mg^{2+}(aq) + 2e^- \]
\[reduction: Ni^{2+}(aq)\rightarrow Ni(s) + 2e^-\]
Mg loses 2 electrons during the reaction to become Mg2+ which means that it is oxidized and found at the anode. Ni2+ gains 2 electrons and is reduced to Ni and found at the cathode. In cell notation, the anode is on the left side and the cathode is on the right. The cathode and anode are the two half cells that are separated by a double line that represents the salt bridge. The solid metal represents the electrode of the reaction and is found at the very end of either end. Within each half cell, reactants are listed first, then products. Each chemical species of a different phase in the reaction is separated by a single vertical line.
\[Mg(s)|Mg^{2+}(aq)||Ni^{2+}(aq)|Ni(s)\]
b. \(2Ag^+(aq)+Cu(s)\rightarrow Cu^{2+}(aq)+2Ag(s)\)
Solution: First identify the oxidation and reduction half reactions:
\[oxidation: Cu(s)\rightarrow Cu^{2+}(aq) + 2e^- \]
\[reduction: 2Ag^+(aq) + 2e^-\rightarrow 2Ag(s) \]
2Ag+ gains 2x1 electrons during the reaction to become 2Ag which means that it is reduced and found at the cathode. Cu loses 2 electrons and is oxidized to Cu2+ and found at the anode. The anode is always at the left of the notation and the cathode is at the right. The cathode and anode are the two half cells that are separated by a double line that represents the salt bridge. The solid metal represents the electrode of the reaction and is found at the very end of either end. Within each half cell, reactants are listed first, then products. Each chemical species in the reaction is separated by a single vertical line.
\[Cu(s)|Cu^{2+}(aq)||Ag^+(aq)|Ag(s)\]
c. \(Mn(s) +Sn(NO_3)_2(aq)\rightarrow Mn(NO_3)_2(aq)+Sn(s)\)
Solution: First identify the oxidation and reduction half reactions:
\[oxdation: Mn(s)\rightarrow Mn^{2+}(aq) + 2e^-\]
\[reduction: Sn^{2+}(aq) + 2e^-\rightarrow Sn(s)\]
In the compound Sn(NO3)2, Sn has a charge of 2+. Sn2+ gains 2 electrons during the reaction to become Sn(s) which means that it is reduced and found at the cathode. Mn loses 2 electrons and is oxidized to Mn2+ and found at the anode. The anode is always at the left of the notation and the cathode is at the right. The cathode and anode are the two half cells that are separated by a double line that represents the salt bridge. The solid metal represents the electrode of the reaction and is found at the very end of either end. Within each half cell, reactants are listed first, then products. Each chemical species in the reaction is separated by a single vertical line.
\[Mn(s)|Mn^{2+}(aq)||Sn^{2+}(aq)|Sn(s)\]
d. \(3CuNO_3(aq)+Au(NO_3)_3(aq)\rightarrow 3Cu(NO_3)_2(aq)+Au(s)\)
Solution: First identify the oxidation and reduction half reactions:
\[oxidation: 3Cu^+(aq)\rightarrow 3Cu^{2+}(aq) + 3e^-\]
\[reduction: Au^{3+}(aq) + 3e^-\rightarrow Au(s) \]
In the compound Au(NO3)3, Au has a charge of 3+. Au3+ gains 3 electrons during the reaction to become Au(s) which means that it is reduced and found at the cathode. The copper in CuNO3 has a charge of +1. Cu loses 1 electron to have a charge of +2 and forms the complex Cu(NO3)2. This is an oxidation half reaction and is found at the anode. The anode is always at the left of the notation and the cathode is at the right. The cathode and anode are the two half cells that are separated by a double line that represents the salt bridge. The solid metal represents the electrode of the reaction and is found at the very end of either end. Within each half cell, reactants are listed first, then products. Each chemical species in the reaction is separated by a single vertical line. Because copper is in ion form, a platinum electrode will be used at the anode. Since Cu+ and Cu2+ are both aqueous, they are separated by a comma instead of a vertical line.
\[Pt(s)|Cu^+(aq), Cu^{2+}(aq)||Au^{2+}(aq)|Au(s)\]
Q. 19.1.10
Would you expect an aqueous manganese(VII) oxide solution to have a pH greater or less than 7.0? Justify your answer.
Manganese(VII) oxide is Mn2O7. Since O has an oxidation state of -2, Mn will have an oxidation number of +7, which is very high. Positive oxidation number refers to how many electrons the element is missing. Due to its high number, it has many spaces in its outer valence shell for lone pair electrons. This means it will act as an excellent lone pair acceptor. According to Lewis Acid-Base theory, an acid is an electron pair acceptor. As per this definition, the aqueous manganese (VII) oxide solution is likely acidic and has a pH less than 7.0.
Q19.3.2
Draw the crystal field diagrams for [Fe(NO2)6]4- and [FeF6]2-. State whether each complex is high spin or low spin, paramagnetic or diamagnetic, and compare Δoct to P for each complex.
Solution:
[Fe(NO2)6]4- has a coordination number of 6 since Fe is bound to 6 monodentate ligands, so its structure will be octahedral. The oxidation state of Fe is +2 because the complex ion has a charge of -4 and the 6 NO2- ions have a total charge of -6 (the inner sphere charge must be equal to the charge of the atom). Fe2+ has 6 valence d electrons. In an octahedral complex, there are 2 eg d-orbitals on top and 3 t2g orbitals on the bottom.
Based on the spectrochemical series, NO2- is a strong ligand, so it will create a larger energy splitting and the complex has a high Δoct, which refers to the energy that it takes for the electron to move into the eg orbitals from the t2g orbitals. This means that it takes more energy for the electron to enter the next energy field (eg) than it takes for the electron to pair (pairing energy = P) with another one in the lower energy field (t2g). The Therefore P<Δoct. This means that the complex ion is low spin and will fill up the lower orbitals first. There are no unpaired electrons, therefore the complex is diamagnetic.
_____ _____ eg
___↓↑__ __↑↓__ __↑↓__ t2g
[FeF6]2- has a coordination number of 6 since Fe is bound to 6 monodentate ligands, so its structure of the ion will be octahedral. The oxidation state of Fe is +4 because the complex ion has a charge of -2 and the 6 F- ions have a charge of -6 (the inner sphere charge must be equal to the charge of the atom). Fe4+ has 4 valence d electrons. The orbital splitting follows the same pattern as above.
Based on the spectrochemical series, F- is a weak ligand, so it will create a lower energy splitting and the complex has a low Δoct, which refers to the energy that it takes for the electron to move into the eg orbitals from the t2g orbitals. This means that it takes less energy for the electron to enter the next energy field (eg) than it takes for the electron to pair (pairing energy = P) with another one in the lower energy field (t2g). The Therefore P>Δoct. This means that the complex ion is high spin and will fill up both levels of orbitals one by one before going back to the t2g orbitals and pairing with the electrons already there. There are 4 unpaired electrons, therefore the complex is paramagnetic.
__↑__ ____ eg
___↑__ __↑__ __↑__ t2g
Q.12.4.2
Use the data to graphically determine the order and rate constant of the following reaction.
In order to determine the rate law for a reaction from a set of data consisting of concentration (or the values of some function of concentration) versus time, make three graphs of the data based on the integrated rate laws of each order reaction.
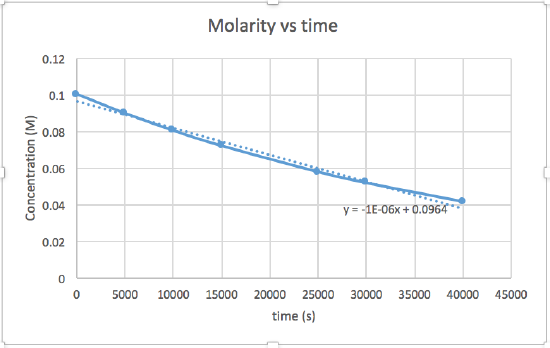
- [concentration] versus time (linear for a zero order reaction)
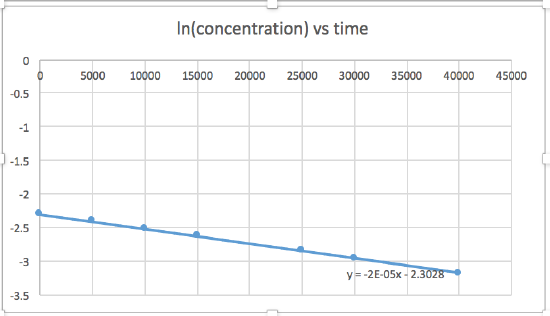
- ln [concentration] versus time (linear for a 1st order reaction)
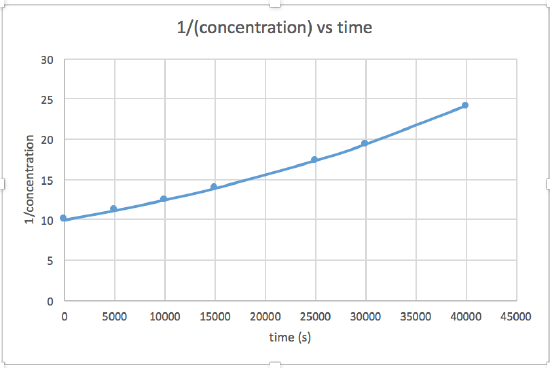
- 1 / [concentration] versus time (linear for a 2nd order reaction)
The graph that is linear indicates the order of the reaction. Then, you can find the correct rate equation:
| zero order reaction | rate = k | (k = - slope of line) |
| 1st order reaction | rate = k[A] | (k = - slope of line) |
| 2nd order reaction | rate = k[A]2 | (k = slope of line) |
In this graph, ln(concentration) vs time is linear, indicating that the reaction is first order.
k=-slope of line
slope= -2.0 x 10-5
k = 2.0 x 10-5
Q.12.7.5
For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed:

A catalyst provides a lower activation energy for a reaction running in the forward direction (reactants →products). Note that the ΔH between the products and reactants will be the same for both the catalyzed and uncatalyzed reactions, so we should analyze the activation energies instead. For both a and b find the height of the activation energy for both graphs and compare. The activation energy is equivalent to the difference between the energy at the highest point and the initial energy level. The reaction that is catalyzed will produce the graph with the lower energy level difference between the reactant energy and the energy of the first activated complex. For both parts identify the graphs with the lower energy.
A. (b) is the catalyzed reaction.
We see that the initial activation energy stays the same across both graphs . However, the second activation energy is lower in graph B than in graph A, making it the catalyzed reaction.
B. (b) is the catalyzed reaction.
The first activation energy in graph B is smaller than the first activation energy in graph A. It is only 5 kJ as opposed to 10 kJ. Therefore, the reaction in graph B will proceed at a faster rate. The second activation energies in both graphs are the same. The answer was originally picked to be A, but its activation energy is higher than the activation energy in graph B, so it is not the catalyzed reaction.
Q 21.4.28
Write a balanced equation for each of the following nuclear reactions:
- mercury-180 decays into platinum-176
Solution: Mercury has 80 protons. Platinum has 78. The change in the number of particles is 2 protons. The mass changes from 180 to 176 which is a change of 4 units. This decay ratio is typical of alpha decay which is a He2+ particle. To balance the equation the masses (upper script) and the number of protons (lower number) must be equal on either side of the arrow. i.e. 180= 4+176 and 80 (Z of Hg)= 78 (z of Pt) +2 (Z of alpha).
\[^{180}_{80}Hg\rightarrow ^4_2α + ^{176}_{78}Pt\]
2. zirconium-90 and an electron are produced by the decay of an unstable nucleus
Solution: An electron has the symbol \(^0_{-1}e^-\). The mass number and atomic number of the products (an electron and zirconium-90) of the decay of an unstable nucleus must add up to the mass number and atomic number of the unstable species. \(^{90}_{40}Zr + ^0_{-1}e = ?\). The sum of the mass numbers 90-0=90 means that the unknown has a mass number of 90 as well. The sum of the atomic numbers 40+-1=39 reveals the number of protons the unstable element had. Yttrium has 39 protons meaning that the nuclear reaction is:
\[^{90}_{39}Y\rightarrow ^{90}_{40}Zr + ^0_{-1}e\]
3. thorium-232 decays and produces an alpha particle and a radium-228 nucleus, which decays into actinium-228 by beta decay
Solution: Throrium 232 has 90 protons and 142 neutrons. If its radioactive decay produces an alpha particle it will also produce a particle with 2 fewer protons and 2 fewer neutrons. If the atomic number is 88 then the element produced is radium-228. If radium-228 produces a beta particle when decaying to actinium-228, that means that it produces an electron (\(^0_{-1}e\)) and one neutron becomes a proton. The mass number will not change but the atomic number will.
\[^{232}_{90}Th\rightarrow ^{228}_{88}Ra + ^4_2α\]
\[^{228}_{88}Ra\rightarrow ^{228}_{89}Ac + ^0_{-1}ß\]
4. neon-19 decays into fluorine-19
Solution: Neon has an atomic number of 10 and Fluorine has an atomic number of 9. Their mass numbers are the same but their atomic numbers are different indicating that the emitted particle has a mass number of 0 and an atomic number of 1 (positron). Therefore the decay is that of beta+ On each side of the arrow the atomic numbers and the mass numbers should be equal.
\[ ^{19}_{10}Ne\rightarrow ^{19}_9F + ^0_1ß\]
Q 20.3.14
For each redox reaction, write the half-reactions and draw the cell diagram for a galvanic cell in which the overall reaction occurs spontaneously. Identify each electrode as either positive or negative.
- Ag(s) + Fe3+(aq) → Ag+(aq) + Fe2+(aq)
Solution: The oxidation half reaction occurs where the anode is. Oxidation occurs with the silver half-reaction because silver loses electrons
\[Oxidation (anode): Ag(s)\rightarrow Ag^+(aq)+e^-\]
The reduction half reaction occurs where the cathode is. Reduction occurs with the Fe3+ because it gains electrons.
\[Reduction (cathode):Fe^{3+}(aq)+e^-\rightarrow Fe^{2+}(aq)
To draw the galvanic cell, begin by drawing the oxidation half cell on the left. Because there is a solid metal in the half reaction. It will serve as the electrode. The metal ion is in the solution and the flow of electrons is away from the anode toward the cathode. The cation (K+) in the salt bridge flows in the same direction as the electrons to balance the charge.
The reduction half cell is drawn on the right side. Because both of the forms of the metal are aqueous ions, the galvanic cell will use a platinum electrode.


2. Fe3+(aq) + 1/2H2(g) → Fe2+(aq) + H+(aq)
Solution: The oxidation half reaction occurs at the anode. Hydrogen loses electrons and is oxidized.
\[Oxidation (anode): 1/2H_2(g)\rightarrow H^+(aq)+e^-\]
The reduction reaction occurs at the cathode. Fe3+ gains electrons and is reduced.
\[Reduction (cathode): Fe^{3+}(aq)+e^-\rightarrow Fe^{2+}(aq)\]
To draw the galvanic cell, first draw the oxidation reaction at the left (where the anode is expected to be). When hydrogen is used in an equation, there is a special electrode that is used that contains a chamber with 1 bar hydrogen gas and a platinum wire to transfer electrons through the electrode and to the other half cell. The electrons and the salt cations travel from left to right.
To draw the reduction reaction, draw a half cell on the right (cathode). Because the metal in the reaction is in aqueous ion form, platinum should be used as the electrode .
Q 20.5.27
Under acidic conditions, ideally any half-reaction with E° > 1.23 V will oxidize water via the reaction O2(g) + 4H+(aq) + 4e-→ 2H2O(l).
- Will aqueous acidic KMnO4 evolve oxygen with the formation of MnO2?
Solution: Yes. The reduction half reactions for MnO4-→MnO2 is displayed in the latimer diagram above. The Eº for the half reaction is 1.70. Since 1.70 is greater than 1.23, KMnO4 will be able to evolve oxygen.
2. At pH 14.00, what is E° for the oxidation of water by aqueous KMnO4 (1 M) with the formation of MnO2?
Solution:
First write the unbalanced equation:
\[MnO_4^-(aq)+2H_2O(l)\rightarrow O_2(g)+4H^+(aq)+MnO_2(s)\]
Then, write and balance the half-reactions:
\[reduction (cathode): 4(3e^-+4H^+(aq)+MnO_4^-(aq)+3e^-\rightarrow MnO_2(s)), E^°_{cell} = 1.70V\]
\[oxidation (anode): 3(2H_2O(l)\rightarrow O_2(g)+4H^+(aq)+4e^-), 1.E^°_{cell} = 1.23V\]
Write the combined, balanced equation:
\[4H^+(aq) + 4MnO_4^-(aq)\rightarrow 4MnO_2(s) + 2H_2O(l) + 3O_2(g)\]
Use the Nernst equation:
where R is the gas constant, T is the temperature in Kelvin, n is the number of transferred electrons, and F is Faraday's constant.
First, we find E°cell, where \[E^°_{cell} = E_{cathode} - E_{anode}\]
\[E^°_{cell} = 1.70V - 1.23V \]
\[E^°_{cell} = 0.47V\]
The concentration of H+ can be found from pH:
\[pH 14 = -log[H^+]\]
\[1*10^{-14}M = [H^+] \]
Q is the reaction quotient:
\[\dfrac{[O_2]^3}{[MnO_4^-]^4[H^+]^4}\]
\[lnQ = [O_2]^3[H^+]^{12}/[MnO_4^-]^4\]
Since specific conditions are not given, we can assume standard conditions of 298K and 1atm for gases.
Then plug into the Nernst equation:
\[E_{cell} = E^°_{cell} + \frac{RT}{NF} * ln\frac{[O_2]^3}{[MnO_4^-]^4[H^+]^4}\]
\[E_{cell} = 0.47V + \frac{(8.314J/mol*K)(298K)}{(12e^-)(96,485 C/mol)} * ln\frac{[1 atm]^3}{[1 M]^4[1*10^{-14}]^4}\]
\[E_{cell} = 0.746V \]
3. At pH 14.00, will water be oxidized if you are trying to form MnO2 from MnO42- via the reaction 2MnO42-(aq) + 2H2O(l) → 2MnO2(s) + O2(g) + 4OH-(aq)? (Problem skipped w Professor Larsen's permission)