Extra Credit 11
- Page ID
- 82767
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 17.1.10
Why must the charge balance in oxidation-reduction reactions?
Solution
An oxidation-reduction reaction occurs when electrons transfer from a substance that is oxidized to one that is being reduced. The oxidant is the species that gains an electron and is reduced in the process, while the reductant is the substance that loses an electron and is oxidized in the process.
The reaction can be described in two half-reactions, one representing the oxidation process and the other one representing the reduction. When the two half-reactions are added, you are left with the overall reaction.
A redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the oxidant.
Like any other balanced chemical equation, the overall process is electrically neutral, the net charge being the same on both sides of the equation and therefore the charge has to be balanced in a redox reaction.
Solution is correct.
To add on: Maintaining charge neutrality is important when using the half reaction method. When we see the amount of electrons transferred, the number of electrons on the oxidation reaction must cancel out the number of electrons on the reduction half reaction. As explained above, the overall reaction will be the addition of these two half reactions, and there will be no electrons in the final product.
Q 19.1.9
Why is the formation of slag useful during the smelting of iron?
Solution
Iron smelting is the ability to extract a pure element from its naturally occurring ores. It includes the reduction of the metallic compound to the metal. With the addition of a compound that forms slag, a substance with low melting point that can be readily separated from the metal, impurities may be removed. The slag is mostly composed of calcium silicate and contains most of the commercially unimportant components of the ore.
Solution is correct.
To add on: Slag is important when extracting iron because it can remove additional elements. For example, in the removal of sulfur, magnesium is used to form MgS shown in the chemical reaction below.
This reaction forms a slag on top of the iron which can then be removed.
Q 19.3.1
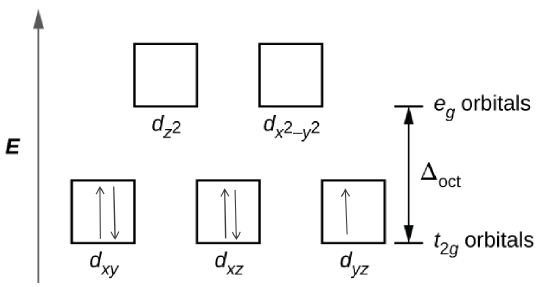
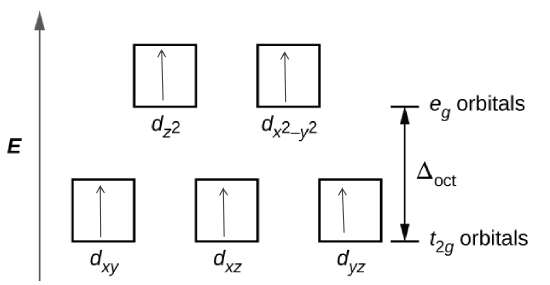
Determine the number of unpaired electrons expected for \([Fe(NO_{2}){_{6}}]^{3-}\) and for \([FeF_{6}]^{3-}\) in terms of crystal field theory.
Information
- The crystal field theory is is a model that describes the breaking of degeneracies of electron orbital states, usually d or f orbitals, due to a static electric field produced by a surrounding charge distribution.
- The degenerate d-orbitals split into two levels, e\(_{g}\) and t\(_{2g}\), in the presence of ligands.
- The energy difference between the two levels is called the crystal-field splitting energy, \(\Delta_{\circ}\).
- After 1 electron each has been filled in the three t\(_{2g}\) orbitals, the filling of the fourth electron takes place either in the e\(_{g}\) orbital or in the t\(_{2g}\), where the electrons pair up. depending on whether the complex is high spin or low spin.
- If the \(\Delta_{\circ}\) value of a ligand is less than the pairing energy (P), then the electrons enter the e\(_{g}\) orbital, but if the \(\Delta_{\circ}\) value of a ligand is more than the pairing energy (P), then the electrons enter the t\(_{2g}\) orbital.
- when the
is less than the pairing energy, the electrons prefer then eg orbitals because there is not enough energy to pair the electrons together. It will be high spin
- when the
is more then the pairing energy, the electrons prefer the t2g because there is enough energy to pair the electrons. It will be low spin.
Solution
Step 1: Determine the oxidation state of the Fe
For \([Fe(NO_{2}){_{6}}]^{3-}\) and \([FeF_{6}]^{3-}\), both \(NO_{2}\) and \(F_{6}\) have a charge of -1. Since there is 6 of them then that means the charge is -6 and in order for there to be an overall charge of -3, Fe has to have a +3 charge.
Step 2: Determine type of ligand
Based on the spectrochemical series we can see that \(NO_{2}^{-}\) is a stronger field ligand than F\(^{-}\), and therefore is a low spin complex because it has a high \(\Delta_{\circ}\) unlike F\(^{-}\) which is a high spin.
Step 3: Draw the crystal field
\([Fe(NO_{2}){_{6}}]^{3-}\)
\([FeF_{6}]^{3-}\)
There is 1 unpaired electron for \([Fe(NO_{2}){_{6}}]^{3-}\), and 5 for \([FeF_{6}]^{3-}\) based on the crystal field theory.
Solution is correct. Just made additional comments.
Q 12.4.1
Describe how graphical methods can be used to determine the order of a reaction and its rate constant from a series of data that includes the concentration of A at varying times.
Solution
When you have a series of data that includes the concentration of A at varying times it's easy to determine both the order of the reaction and the rate constant.
We assume that the order of the reaction is zero, first or second.
With the rate law of a given order we are able to calculate the integrated equation, the concentration-time equation.
Zero Order Reaction
Rate law: Rate=k
If we were to integrate we get [A]t - [A]0 = kt, where where [A]t is the concentration of reactant A at time t, and [A]0 is the concentration of reactant A at the beginning of the reaction.
First Order Reaction
Rate law: Rate= k[A]
If we were to integrate we get \(ln\frac{[A]_{t}}{[A]_{0}}\) Wrong, should be
Second Order Reaction
Rate law: Rate= k [A\(^{2}\)]
If we integrate we get \(\frac{1}{[A_{t}]}=kt+\frac{1}{[A]_{0}}\)
In order to determine the order of the reaction we plot three different graphs, concentration vs. time, ln( concentration) vs. time, and \(\frac{1}{[A]}\) vs. time. When we plot the data we will see that one of them is linear and if its the concentration vs. time one, then the order is zero, if its ln(concentration) vs. time then the reaction is first order, if the its the one of \(\frac{1}{[A]}\) vs. time ,then the reaction is second order.
Once you have determined the reaction order, you can easily get the rate constant by obtaining the slope of the graph.
In addition, the slope of a second order reaction should be positive compared to a zero or first order reaction which is negative.
Q 12.7.4
For each of the following pairs of reaction diagrams, identify which pair is catalyzed:
a.
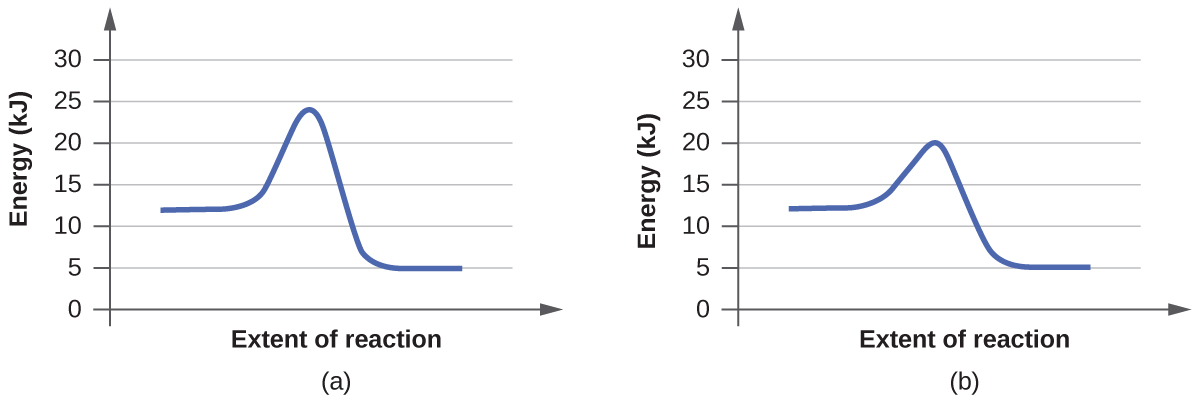
b.

Solution
A catalyst is a substance which increases the rate of reaction by lowering the activation energy but it remains unchanged in the reaction
In order to answer the question you have to determine which graph there was a lowering of the transition state of a catalyst
In the case of a. we see that reaction (b) has a lower energy of the transition state and in the case of b. (b) also has the lowest transition state which indicates the presence of a catalyst.
Solution is correct.
Addition comments: We can also conclude the fact that a catalyst can increase the rate of both the forward and backwards reaction. Since both (b) in each pair exhibit this decrease in required energy, we can say that (b) is the reaction with the catalyst.
Q 21.4.27
Write a balance equation for each of the following nuclear reactions:
a. bismuth-212 decays into polonium-212
b. beryllium-8 and a positron are produced by the decay of an unstable nucleus
c. neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
d. strontium -90 decays into yttrium-90
Information
In a nuclear decay reaction, an unstable nucleus emits radiation and is transformed into the nucleus of one or more other elements.
In a nuclear decay reaction the relationship between the total mass number of the reactants and the total mass number of the products is that they are both sides have to equal each other. An important concept from the law of conservation of mass.
In order to be able to answer the question we have to know the three different types of radiation.
Alpha Radiation/Decay
- Sometimes represented by Greek letter \(\alpha\)
- It's composed of two protons and two neutrons and its a nucleus of the element helium $$_{4}^{2}\textrm{He }^{2+}$$ as shown where 4 is the total mass number, 2 is the atomic number and 2+ is the charge.
- When a radioactive atom emits an alpha particle, the atomic mass of a an element decreases by around 4 amu due to the loss of 4 nucleons.
- The atomic number of an element goes down by 2, because it loses two protons and this means that it becomes a new element.
Beta Radiation
The emission of a beta particle will not change the mass of the resulting element but it will change the atomic number by adding a charge to the nucleus. There are two kinds of beta radiation and they include electron and positron
Electron
- they are just electrons from the nucleus and are represented as \(\beta^-\) or \(_{-1}^{0}\textrm{e}\)
- Beta emission is also includes the emission of an electron antineutrino, which shares the momentum and energy of the decay.
- The resulting ion is also charged because of the added proton
Positron
- The emission of a positron (an antielectron) is also called a beta decay and represented as \(\beta^+\) or \(_{+1}^{0}\textrm{e}\)
- The positron is accompanied by a neutrino which is a massless and charmless particle.
- We must also emphasize that the mass number for beta radiation does not change.
Gamma Radiation/Decay
- A gamma ray is represented as \(_{0}^{0}\textrm{\gamma}\)
- Gamma ray emission occurs with \(\alpha\) and \(\beta\) emission.
- Gamma rays have no charge or mass
- Gamma ray emission occurs because the nucleus is often unstable after \(\alpha\) and \(\beta\) decay.
a. bismuth-212 decays into polonium-212
Step 1: Determine the atomic and mass number of the elements.
Bismuth-212 has an atomic number of 83 so we write it as, \(_{83}^{212}\textrm{Bi}\), Polonium-212 has an atomic number of 84, \(_{84}^{212}\textrm{Po}\)
Step 2: Determine the type of radiation that is emitted by the reaction.
The atomic number increased by one so we can determine that it is emitting a beta particle, \(_{-1}^{0}\textrm{e}\)
Step 3: Write equation
$$_{83}^{212}\textrm{Bi}\rightarrow _{84}^{212}\textrm{Po}+_{-1}^{0}\textrm{e}$$
Step 4: Make sure that both sides are balance, equal to each other
Mass number 212 → 212 + 0
Atomic number 83 → 84 + -1
Solution
$$_{83}^{212}\textrm{Bi}\rightarrow _{84}^{212}\textrm{Po}+_{-1}^{0}\textrm{e}$$
b. beryllium-8 and a positron are produced by the decay of an unstable nucleus
Step 1: Determine atomic number
Beryllium: \(_{4}^{8}\textrm{Be} \) Positron: \(_{+1}^{0}\textrm{e}\)
Step 2: Find unknown nucleus
Since its a beta decay (positron), you know that the atomic number increases by 1 and there is no change in the molar mass, therefore atomic number= 1+ 4= 5, which is boron.
Unknown element is \(_{5}^{8}\textrm{B} \)
Step 3: Write equation
\(_{5}^{8}\textrm{B}\rightarrow _{4}^{8}\textrm{Be}+ _{+1}^{0}\textrm{e}\)
Step 4: Check
Mass number 8 → 8 + 0
Atomic number 5 → 4 + 1
Solution
$$_{5}^{8}\textrm{B}\rightarrow _{4}^{8}\textrm{Be}+ _{+1}^{0}\textrm{e}$$
c. neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
Step 1: Write equation given
You are told that neptumin-239 forms from uranium-238 an a neutron, \(_{0}^{1}\textrm{n} \). You also know that its a beta emission, electron, because the atomic number goes from 92 in uranium to 93 in neptunium.
$$_{92}^{238}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{93}^{239}\textrm{Np}+_{-1}^{0}\textrm{e}$$
Step 2: Account for the spontaneous part
$$_{93}^{239}\textrm{Np}+\rightarrow _{94}^{239}\textrm{Pu}+_{-1}^{0}\textrm{e}$$
Solution
$$_{92}^{238}\textrm{U}+_{0}^{1}\textrm{n}\rightarrow _{93}^{239}\textrm{Np}+_{-1}^{0}\textrm{e}, _{93}^{239}\textrm{Np}+\rightarrow _{94}^{239}\textrm{Pu}+_{-1}^{0}\textrm{e}$$
d. strontium -90 decays into yttrium-90
Step 1: Find atomic number
Strontium-90 has an atomic number of 38: \(_{38}^{90}\textrm{Sr}\) Yttrium-90 has an atomic number of 39: \(_{39}^{90}\textrm{Y}\)
Step 2: Determine the type of radioativity
Since the mass number is constant and the atomic number changes by one its a beta emission, specifically a \(_{-1}^{0}\textrm{e}\)
Step 3: Write equation
$$_{38}^{90}\textrm{Sr}+\rightarrow _{39}^{90}\textrm{Y}+_{-1}^{0}\textrm{e}$$
Step 4: Check
Mass number 90 → 90 + 0
Atomic number 38 → 39 + -1
Solution
$$_{38}^{90}\textrm{Sr}+\rightarrow _{39}^{90}\textrm{Y}+_{-1}^{0}\textrm{e}$$
Solutions are correct.
Q 20.3.13
For each of galvanic cell represented by these cell diagrams, determine the spontaneous half-reactions and the overall reaction. Indicate which reaction occurs at the anode and which occurs at the cathode.
a. \(Zn(s)\mid Zn^{2+}(aq)\parallel H^{+}(aq)\mid H_{2}(g),Pt(s)\)
b. \(Ag(s)\mid AgCl(s)\mid Cl^{-}(aq)\parallel H^{+}(aq)\mid H_{2}(g)\mid Pt(s)\)
c. \(Pt(s)\mid H_{2}(g)\mid H^{+}(aq)\parallel Fe^{2+}(aq)\mid Fe^{3+}(aq)\mid Pt(s)\)
Information
- A galvanic cell is a type of electrochemical cell, which is an apparatus that is used to generate electricity from a spontaneous redox reaction or that uses electricity to drive a spontaneous redox reaction.
- Specifically a galvanic cell uses the energy released during a spontaneous redox reaction (\(\Delta\)G < 0) to generate electricity.
- Galvanic cells transform chemical energy into electrical energy that can be used to do work.
- It contains two electrodes, which are solid metals connected to an external circuit that provides an electrical connection between the two parts of the system.
- The oxidation half-reaction occurs at the one electrode, called the anode, an the reduction half-reaction occurs at the other, called the cathode. When the circuit is close, electrons flow from the anode to the cathode, with the help of a salt bridge to maintain the system's electrical neutrality.
- In order to get the overall reaction, add the two half reactions together so that the electrons cancel each other, if they don't then multiply or divide until its possible to cancel them out.
- The potential (E\(_{cell}\)) of the cell, measured in volts, V, is the difference in electrical potential between the two half-reactions and is related to the energy needed to move a charged particle in an electric field. $$E^{\circ}_{cell} = E^{\circ}_{cathode} - E^{\circ}_{anode}$$
- If the \(E^{\circ}_{cell}\) is positive, the reaction will occur spontaneously under standard conditions but if its negative then the reaction is not spontaneous under standard conditions, although it will proceed spontaneously in the opposite direction
- The standard potential is given for reduction half-reactions and the sign needs to be flip if its an oxidation reaction
Solution
a. \(Zn(s)\mid Zn^{2+}(aq)\parallel H^{+}(aq)\mid H_{2}(g),Pt(s)\)
Step 1: Identify the oxidation half-reaction and the reduction half-reaction.
In the oxidation half-reaction, Zn is oxidized to Zn\(^{2+}\)
$$Zn(s) → Zn^{2+}(aq) + 2e^{-}$$
In the reduction half-reaction, H\(^{+}\) is being reduced to H
$$H^{+}(aq) + e^{-} → H(g)$$
Step 2: Identify the cathode and anode
Because the reduction reaction occurs at the Pt electrode, it is the cathode an since the oxidation reaction occurs at the zinc electrode then its the anode.
Step 3: Identify the spontaneous half-reaction
The \(E^{\circ}_{cell}\) for the oxidation half-reaction is 0.763 V and for the reduction half-reaction it's 0.00 V
Therefore, \(E^{\circ}_{cell}\)= 0.00-0.763= -0.763V and since its negative, then the reaction of Zn/Zn\(^{2+}\) is not spontaneous.
Anode: \(Zn(s) → Zn^{2+}(aq) + 2e^{-}\)
Cathode: \(H^{+}(aq) + e^{-} → H(g)\) Spontaneous
Overall Reaction: Zn(s) + 2H\(^{+}\)(aq) → Zn\(^{2+}\)(aq) + H\(_{2}\)(g)
b. \(Ag(s)\mid AgCl(s)\mid Cl^{-}(aq)\parallel H^{+}(aq)\mid H_{2}(g)\mid Pt(s)\)
Step 1: Identify the oxidation and reduction half-reaction
In the oxidation half-reaction, H\(_{2}\) is being oxidized to H\(^{+}\)
$$ H_{2}(g)→ 2H^{+}(aq) + 2e^{-}$$
In the reduction half-reaction, AgCl is being reduced to Ag and Cl\(^{-}\)
$$AgCl(s) + e^{-} → Ag(s) + Cl^{-}(aq)$$
Step 2: Identify the cathode and anode
Because the reduction reaction occurs at the AgCl electrode, it is the cathode an since the oxidation reaction occurs at the Pt electrode then its the anode.
Step 3: Identify the spontaneous half-reaction
The \(E^{\circ}_{cell}\) for the oxidation half-reaction is 0.00 V and for the reduction half-reaction it's 0.2223 V
Therefore, \(E^{\circ}_{cell}\)= 0.2223-0.00= 0.2223 V and since its positive, then the reaction of AgCl/Ag Cl\(^{-}\) is spontaneous.
Anode: \(H_{2}(g) → 2H^{+}(aq) + 2e^{-}\)
Cathode: \(AgCl(s) + e^{-} → Ag(s) + Cl^{-}(aq)\) Spontaneous
Overall Reaction: \(AgCl(s) + H_{2}(g)→ 2H^{+}(aq) + Ag(s) + Cl^{-}(aq)\)
c. \(Pt(s)\mid H_{2}(g)\mid H^{+}(aq)\parallel Fe^{2+}(aq)\mid Fe^{3+}(aq)\mid Pt(s)\)
Step 1: Identify the oxidation half-reaction and the reduction half-reaction.
In the oxidation half-reaction, H\(_{2}\) is oxidized to H\(^{+}\)
$$H_{2}(g) → 2H^{+}(aq) + 2e^{-}$$
In the reduction half-reaction, Fe\(^{3+}\) is being reduced to Fe\(^{2+}\)
$$Fe^{3+}(aq) + e^{-} → Fe^{2+}(aq)$$
Step 2: Identify the cathode and anode
Because the reduction reaction occurs at the Fe\(^{3+}\) electrode, it is the cathode an since the oxidation reaction occurs at the H\(_{2}\) electrode then its the anode.
Step 3: Identify the spontaneous half-reaction
The \(E^{\circ}_{cell}\) for the oxidation half-reaction is 0.00 V and for the reduction half-reaction it's -0.447 V
Therefore, \(E^{\circ}_{cell}\)= -0.447 - 0.00= -0.447 V and since its negative, then the reaction of Fe\(^{3+}\)/Fe\(^{2+}\) is not spontaneous.
Anode \(H_{2}(g) → 2H^{+}(aq) + 2e^{-}\) Spontaneous
Cathode \(Fe^{3+}(aq) + e^{-} → Fe^{2+}(aq)\)
Overall Reaction \(2Fe^{3+}(aq) + H_{2}(g) → 2H^{+}(aq) + 2Fe^{2+}(aq)\)
Solutions are correct.
Q 20.5.26
Ideally, any half-reaction with the \(E^{\circ }\) > 1.23 V will oxidize water as a result of the half-reaction \(O_{2}(g)+4H^{+}(aq)+4e^{-}\rightarrow 2H_{2}O(l)\).
a. Will \(FeO_{4}^{2-}\) oxidize water if the half-reaction for the reduction of Fe(IV)→ Fe(III) is \(FeO_{4}^{2-}(aq)+8H^{+}(aq)+3e^{-}\rightarrow Fe^{3+}(aq)+4H_{2}O\); \(E^{\circ }\)= 1.9 V?
b. What is the highest pH at which this reaction will proceed spontaneously if [\(Fe^{3+}\)] = [\(FeO_{4}^{2-}\)]= 1.0 M and \( P_{O_{2}}\) = 1.0 atm?
Solution
a. If a reduced species is harder to oxidize than water itself, water will just get oxidized instead. The \(E^{\circ }\) for the reduction of Fe(IV)→ Fe(III) is 1.9 V and since it is greater than 1.23 V, it will therefore oxidize water.
b. The reaction will proceed spontaneously in the forward direction if \(E^{\circ }\) > 0. We can use the Nernst equation to determine -log(H+) and therefore get the pH. We are told that [\(Fe^{3+}\)] = [\(FeO_{4}^{2-}\)]= 1.0 M and \( P_{O_{2}}\) = 1.0 atm, which we can use to determine K. The Nernst equation is $$E_{cell}= E_{cell}^{o} - (\frac{0.0591 V}{n})logK$$
We can plug in the information for K and get $$E_{cell}= E_{cell}^{o} - (\frac{0.0591 V}{3})log(\frac{[Fe^{3+}][P_{O_{2}}]}{[FeO_{4}^{2-}][H^{+}]^{8}}$$The question is asking for the highest pH in which the reaction will proceed spontaneously so we can st E\(_{cell}\)= 0
$$0 = 1.9 V - (\frac{0.0591 V}{3})log(\frac{[Fe^{3+}][P_{O_{2}}]}{[FeO_{4}^{2-}][H^{+}]^{8}}$$
We can then plug in the values for [\(Fe^{3+}\)] = [\(FeO_{4}^{2-}\)]= 1.0 M and \( P_{O_{2}}\) = 1.0 atm and solve for -log[H\(^{+}\)]
$$0 = 1.9 V - (\frac{0.0591 V}{3})log(\frac{[1][1]}{[1][H^{+}]^{8}}$$
$$96.4= log(\frac{1}{[H^{+}]^{8}} = log[H^{+}]^{-8}= (-8) log [H^{+}]$$
$$12.06=-log [H^{+}]$$
$$pH=12.06$$
Solutions are correct.