Extra Credit 8
- Page ID
- 82864
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.7
Balance the following in basic solution:
- \(\ce{SO3^2-}(aq)+\ce{Cu(OH)2}(s)⟶\ce{SO4^2-}(aq)+\ce{Cu(OH)}(s)\)
- \(\ce{O2}(g)+\ce{Mn(OH)2}(s)⟶\ce{MnO2}(s)\)
- \(\ce{NO3-}(aq)+\ce{H2}(g)⟶\ce{NO}(g)\)
- \(\ce{Al}(s)+\ce{CrO4^2-}(aq)⟶\ce{Al(OH)3}(s)+\ce{Cr(OH)4-}(aq)\)
S17.1.7
solution is correct - Phase II
1) \(\ce{SO3^2-}(aq)+\ce{Cu(OH)2}(s)⟶\ce{SO4^2-}(aq)+\ce{Cu(OH)}(s)\)
Step 1: Separate into two half reactions; reduction and oxidation. Reduction reactions gain electrons while oxidation reactions lose an electron.
\(\ce{SO3^2-}(aq)⟶\ce{SO4^2-}(aq)\) (oxidation half reaction) This is the oxidation reaction because S(sulfur) goes from a +4 oxidation state to a +6 oxidation state, thus losing 2 electrons in the process.
\(\ce{Cu(OH)2}(s)⟶\ce{Cu(OH)}(s)\) (reduction half reaction) This is the reduction reaction because Cu goes from a +2 oxidation state to a +1 oxidation state, thus gaining 1 electron in the process.
Step 2: Balance the two reactions.
To balance the oxidation reaction we must use water to balance the oxygens:
\(\ce{H2O}(l)+\ce{SO3^2-}(aq)⟶\ce{SO4^2-}(aq)\)
To balance the reduction reaction we must add OH^- to the right side:
\(\ce{Cu(OH)2}(s)⟶\ce{Cu(OH)}(s)+\ce{OH^-}(aq)\)
Step 3: Balance the hydrogen with protons(H+):
This is only necessary for the oxidation reaction:
\(\ce{H2O}(l)+\ce{SO3^2-}(aq)⟶\ce{SO4^2-}(aq)+{2H^+}(aq)\)
Step 4: Balance the charges with electrons:
Since Sulfur oxidizes from +4 to +6 we must add 2e- to the right side of the oxidation reaction:
\(\ce{H2O}(l)+\ce{SO3^2-}(aq)⟶\ce{SO4^2-}(aq)+{2H^+}(aq)+{2e-}\)
For the reduction reaction we only add 1e- because Cu reduces from 2+ to 1+:
\(\ce{Cu(OH)2}(s)+{1e-}⟶\ce{Cu(OH)}(s)+\ce{OH^-}(aq)\)
Step 5: Scale the reactions so they have the same number of electrons:
We must multiply the reduction reaction by 2 so the it has 2e- like the oxidation reaction:
\(\ce{2Cu(OH)2}(s)+{2e-}⟶\ce{2Cu(OH)}(s)+\ce{2OH^-}(aq)\)
Step 6:Add reactions and cancel out the electrons:
\(\ce{H2O}(aq)+\ce{SO3^2-}(aq)+\ce{2Cu(OH)2}(s)⟶\ce{SO4^2-}(aq)+{2H^+}(aq)+\ce{2Cu(OH)}(s)+\ce{2OH^-}(aq)\)
Step 7: Add OH- to balance H+. There are 2 net protons in this equation, so add 2 OH- ions to each side.
\(\ce{H2O}(aq)+\ce{SO3^2-}(aq)+\ce{2Cu(OH)2}(s)+\ce{2OH^-}(aq)⟶\ce{SO4^2-}(aq)+{2H^+}(aq)+\ce{2Cu(OH)}(s)+\ce{4OH^-}(aq)\)
Step 8:Combine OH- ions and H+ ions that are present on the same side to form water.
\(\ce{H2O}(aq)+\ce{SO3^2-}(aq)+\ce{2Cu(OH)2}(s)+\ce{2OH^-}(aq)⟶\ce{SO4^2-}(aq)+\ce{2Cu(OH)}(s)+\ce{2OH^-}(aq)+\ce{2H2O}(aq)\)
Cancel and Combine terms:
\(\ce{SO3^2-}(aq)+\ce{2Cu(OH)2}(s)⟶\ce{SO4^2-}(aq)+\ce{2Cu(OH)}(s)+\ce{H2O}(aq)\)
2)\(\ce{O2}(g)+\ce{Mn(OH)2}(s)⟶\ce{MnO2}(s)\)
Step 1:Separate into two half reactions; reduction and oxidation. Reduction reactions gain electrons while oxidation reactions lose an electron.
\(\ce{Mn(OH)2}(s)⟶\ce{MnO2}(s)\) This is the oxidation half reaction because Mn goes from an oxidation state of +2 to an oxidation state of +4, thus losing two electrons in the process.
\(\ce{O2}(g)⟶\ce{MnO2}(s)\) This is the reduction half reaction because Oxygen goes from an oxidation state of 0 to -4, thus gaining four electrons in the process.
Step 2: Balance the two half reactions:
In order to balance the oxidation reaction we must add 2 H+ to the right side to balance out the left side:
\(\ce{Mn(OH)2}(s)⟶\ce{MnO2}(s)+{2H^+}(aq)\)
To balance the reduction reaction all we need to do is add a Mn to the left side:
\(\ce{O2}(g)+{Mn}(s)⟶\ce{MnO2}(s)\)
Step 3: Balance the charges of the half reactions with electrons:
For the oxidation reaction, we need to add 2 electrons to the right side because Mn is oxidized from 2+ to 4+:
\(\ce{Mn(OH)2}(s)⟶\ce{MnO2}(s)+{2H^+}(aq)+{2e^-}\)
For the reduction half reaction, we must add 4 electrons to the left side because Oxygen is reduced from 0 to -4:
\(\ce{O2}(g)+{Mn}(s)+{4e^-}⟶\ce{MnO2}(s)\)
Step 4: Scale the reactions so that they both have the same number of electrons.
In order to balance the number of electrons for both half reactions, we must multiply the oxidation reaction by 2 so that it has 4 electrons just like the reduction half reaction. We leave the reduction half reaction how it is.
\(\ce{2Mn(OH)2}(s)⟶\ce{2MnO2}(s)+{4H^+}(aq)+{4e^-}\)
\(\ce{O2}(g)+{Mn}(s)+{4e^-}⟶\ce{MnO2}(s)\)
Step 5: Add both have reactions and cancel out the electrons:
\(\ce{2Mn(OH)2}(s)+\ce{O2}(g)+{Mn}(s)⟶\ce{3MnO2}(s)+{4H^+}(aq)\)
Step 6:Add OH- to balance H+. There are 4 net protons in this equation, so add 4 OH- ions to each side.
\(\ce{2Mn(OH)2}(s)+\ce{O2}(g)+{Mn}(s)+{4OH^-}(aq)⟶\ce{3MnO2}(s)+{4H^+}(aq)+{4OH^-}(aq)\)
Step 7:Combine OH- ions and H+ ions that are present on the same side to form water.
\(\ce{2Mn(OH)2}(s)+\ce{O2}(g)+{Mn}(s)+{4OH^-}(aq)⟶\ce{3MnO2}(s)+\ce{4H2O}(aq)\) This is your answer.
3)\(\ce{NO3-}(aq)+\ce{H2}(g)⟶\ce{NO}(g)\)
Step 1: Separate into two half reactions; reduction and oxidation. Reduction reactions gain electrons while oxidation reactions lose an electron.
\(\ce{H2}(g)⟶\ce{2H^+}(aq)\) This is the oxidation reaction because hydrogen loses 2 electrons in the process. We must balance the H2 on the reactants side with 2H+ on the product side.
\(\ce{NO3^-}(aq)⟶\ce{NO}(g)\) This is the reduction half reaction because Nitrogen goes from an oxidation state of +5 to +2, gaining 3 electrons in the process.
Step 2: Balance the two half reactions:
The oxidation half reactions is already balanced from the previous step.
In order to balance the reduction half reaction we need to add 3 water molecules to the product side in order to balance the oxygen on the reactant side. Since we add 3 water molecules, the reactant side is now missing 6 H atoms. So we add 6H+ to the left side. The reduction half reaction now looks like this:
\(\ce{NO3^-}(aq)+\ce{6H^+}(aq)⟶\ce{NO}(g)+\ce{H2O}(l)\)
Step 3: Balance the charge of both reactions using electrons:
For the oxidation reaction must add 2 electrons to the right side because Hydrogen loses 2 electrons in the process.
\(\ce{H2}(g)⟶\ce{2H^+}(aq)+\ce{2e^-}\)
For the reduction half reaction we must add 5 electrons tot he left side because nitrogen gains 5 electrons in the process.
\(\ce{NO3^-}(aq)+\ce{6H^+}(aq)+\ce{5e^-}⟶\ce{NO}(g)+\ce{H2O}(l)\)
Step 4: Scale the two half reactions in order to cancel out the electrons:
In order to do this we must find a common factor of 2 electrons and 5 electrons. We find that it is 10. So we multiply the oxidation half reaction by a factor of 5 and the reduction half reaction by a factor of 2. We get the following:
\(\ce{5H2}(g)⟶\ce{10H^+}(aq)+\ce{10e^-}\)
\(\ce{2NO3^-}(aq)+\ce{12H^+}(aq)+\ce{10e^-}⟶\ce{2NO}(g)+\ce{2H2O}(l)\)
Step 5: Add both half reactions and cancel out the electrons:
\(\ce{5H2}(g)+\ce{2NO3^-}(aq)+\ce{12H^+}(aq)⟶\ce{2NO}(g)+\ce{10H^+}(aq)+\ce{6H2O}(l)\)
Step 6: Now cancel out the H+ on both sides and you have your final answer as follows:
\(\ce{5H2}(g)+\ce{2NO3^-}(aq)+\ce{2H^+}(aq)⟶\ce{2NO}(g)+\ce{6H2O}(l)\)
4)\(\ce{Al}(s)+\ce{CrO4^2-}(aq)⟶\ce{Al(OH)3}(s)+\ce{Cr(OH)4-}(aq)\)
Step 1: Separate into two half reactions; reduction and oxidation. Reduction reactions gain electrons while oxidation reactions lose an electron.
\(\ce{Al}(s)⟶\ce{Al(OH)3}(s)\) This is the oxidation half reaction because Al goes from an oxidation state of 0 to +3.
\(\ce{CrO4^2-}(aq)⟶\ce{Cr(OH)4^-}(aq)\) This is the reduction hafl reaction because Cr goes form and oxidation state of +7 to +5.
Step 2: Balance the half reactions.
In order to balance the oxidation half reaction we must add 3 OH- molecules to the reactants.
\(\ce{Al}(s)+\ce{3OH^-}(aq)⟶\ce{Al(OH)3}(s)\)
In order to balance the reduction half reaction we must balance the Hydrogen on the left side by adding 4 water molecules. By doing that we now have an uneven number of oxygen and hydrogen molecules on the right side, so we must add 4 OH-.
\(\ce{CrO4^2-}(aq)+\ce{4H2O}(l)⟶\ce{Cr(OH)4^-}(aq)+\ce{4OH^-}(aq)\)
Step 3: Add electrons to both half reactions to balance the charges.
\(\ce{Al}(s)+\ce{3OH^-}(aq)⟶\ce{Al(OH)3}(s)+\ce{3e^-}\)
\(\ce{CrO4^2-}(aq)+\ce{4H2O}(l)+\ce{2e^-}⟶\ce{Cr(OH)4^-}(aq)+\ce{4OH^-}(aq)\)
Step 4: Multiply by a common factor to cancel the electrons.
In order to cancel out the electrons we must find a common multiple. The common multiple is 6. So we multiply the oxidation half reaction by a factor of 2 and the reduction half reaction by a factor of 3.
\(\ce{2Al}(s)+\ce{6OH^-}(aq)⟶\ce{2Al(OH)3}(s)+\ce{6e^-}\)
\(\ce{3CrO4^2-}(aq)+\ce{12H2O}(l)+\ce{6e^-}⟶\ce{3Cr(OH)4^-}(aq)+\ce{12OH^-}(aq)\)
Step 5: Add both half reactions and cancel out electrons:
\(\ce{2Al}(s)+\ce{6OH^-}(aq)+\ce{3CrO4^2-}(aq)+\ce{12H2O}(l)⟶\ce{2Al(OH)3}(s)+\ce{3Cr(OH)4^-}(aq)+\ce{12OH^-}(aq)\)
Step 6: Cancel out common terms. The following is the final answer:
\(\ce{2Al}(s)+\ce{3CrO4^2-}(aq)+\ce{12H2O}(l)⟶\ce{2Al(OH)3}(s)+\ce{3Cr(OH)4^-}(aq)+\ce{6OH^-}(aq)\)
Q19.1.6
Which of the following is the strongest oxidizing agent: \(\ce{VO4^3-},\ce{CrO4^2-}, or \ce{MnO4^-}\)
S19.1.6
In order to do this problem it would be beneficial if we looked at the table of standard reduction potentials: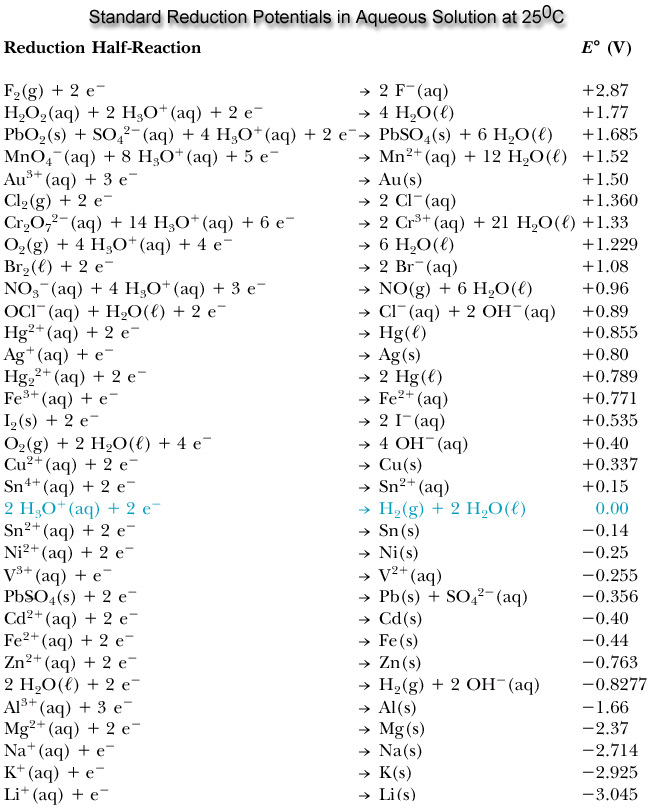
Phase II - (additional comment): The species with the highest reduction potential is the strongest oxidizing agent.
So we see that \(\ce{MnO4^-}\) has the highest reduction potential.
Q19.2.8
Specify whether the following complexes have isomers.
- tetrahedral [Ni(CO)2(Cl)2]
- trigonal bipyramidal [Mn(CO)4NO]
- [Pt(en)2Cl2]Cl2
S19.2.8
Phase II: Answer is correct
To know how to do this problem we must know about the different types of isomers. There are what we call structural isomers that consist of ionization, coordination, and linkage isomers. However, we do no need to know these forms of isomers to solve this problem. To solve this problem we must know about stereoisomers. Stereoisomers consist of geometric and optical isomers. Geometric isomers are two or more coordination compounds that contain the same formula and bonds, but have different spatial ordering of the atoms. Within geometric isomers there are cis and trans isomers. Cis isomers create 90 degree angles between atoms. Trans isomers are 180 degrees from each other, so directly across from one another. Cis and trans isomers can occur in square planar and octahedral complexes. Also within the geometric isomers are mer and fac isomer. A mer isomer contains a cis and fac isomer while a fac isomer contains all cis isomers. Optical isomers two compounds which contain the same number and kinds of atoms, and bonds, and spacial arrangement, with non-superimposable mirror images. When thinking about optical isomers think of places your hands on top of each other. They are mirror images that are non-superimposable.
So,
1. Cannot have any isomers because it is a tetrahedral complex, not square planar.
2. Cannot have any isomers because it has NO atoms and only one CO molecule.
3. The 2 Cl2 molecules can either be cis or trans isomers. This is also a optical isomer.
Q12.3.21
The annual production of HNO3 in 2013 was 60 million metric tons Most of that was prepared by the following sequence of reactions, each run in a separate reaction vessel.
- 4NH3(g)+5O2(g)⟶4NO(g)+6H2O(g)4NH3(g)+5O2(g)⟶4NO(g)+6H2O(g)
- 2NO(g)+O2(g)⟶2NO2(g)2NO(g)+O2(g)⟶2NO2(g)
- 3NO2(g)+H2O(l)⟶2HNO3(aq)+NO(g)3NO2(g)+H2O(l)⟶2HNO3(aq)+NO(g)
The first reaction is run by burning ammonia in air over a platinum catalyst. This reaction is fast. The reaction in equation (c) is also fast. The second reaction limits the rate at which nitric acid can be prepared from ammonia. If equation (b) is second order in NO and first order in O2, what is the rate of formation of NO2 when the oxygen concentration is 0.50 M and the nitric oxide concentration is 0.75 M? The rate constant for the reaction is 5.8 × 10−6 L2/mol2/s.
S12.3.21
The rate of the reaction is the speed or rate of change of concentration with time at which the reaction proceeds in a particular direction
The reaction that dictates the rate of the reaction is equation b:
\(\ce{2NO}(g)+\ce{O2}⟶\ce{2NO2}(g)\)
Equation B is second order with respect to NO and first order with respect to oxygen. So the rate equation will be Rate=k[NO]^2[O2]
The values are as follows:
Rate constant k: 5.86x10^-6 (mol^-2)(s^-1)
[NO]=0.75M
[O2]=0.50M
Phase II: (additional comment) - Substitute these values into the rate law equation: R = (5.86x10^-6)(.75)^2(.50)= 1.631x10^-6 M(s^-1). Considering the stoichiometry of these reactions, the rate expression is written as:

So the rate of formation for NO2 can be calculated as follows:

Q12.7.1
Account for the increase in reaction rate brought about by a catalyst.
S12.7.1
A catalyst works by lowering the energy of activation. This enhances the rate of forward and backward reaction. The catalyst forms an intermediate with the reactant(s) in the initial step of the reaction and is released in during product formation. A catalyst can not affect the enthalpies or the Gibbs energies of the reactants and products. It increases the rate of the approach to equilibrium, but can not change the change the equilibrium constant. A catalyst is a substance which speeds up a reaction, but is chemically unchanged at the end of the reaction. When the reaction has finished, you would have exactly the same mass of catalyst as you had at the beginning. The general mode of action for a catalyst is to provide a mechanism by which the reactants can unite more readily by taking a path with a lower reaction energy. The rates of both the forward and the reverse reactions are increased, leading to a faster achievement of equilibrium.
Q21.4.24
A 74Be atom (mass = 7.0169 amu) decays into a 73Li atom (mass = 7.0160 amu) by electron capture. How much energy (in millions of electron volts, MeV) is produced by this reaction?
S21.4.24
Phase II: Answer is correct.
First, we must subtract the mass of the Li atom from the Be atom:
(7.0169amu)-(7.0160amu)=.0009amu
For this problem we need to use the formula, Delta(E)= Delta(m) x c^2
So,
=(.0009amu)(1.6605x10^-27 kg amu^-1)(3.00x10^8 m/s)^2
=1.345x10^-13
Therefore,
Energy= (1.345x10^-13)(6.022x10^23)=8.01x10^10
This is equal to 0.8MeV
Q20.3.11
Sulfate is reduced to HS− in the presence of glucose, which is oxidized to bicarbonate. Write the two half-reactions corresponding to this process. What is the equation for the overall reaction?
S20.3.11
Phase II: Answer is correct.
In order to complete this problem we first must write out the equation we will be dealing with:
\(\ce{SO4^2-}(aq)+\ce{C6H12O6}(aq)⟶\ce{HS^-}(aq)+\ce{HCO3^-}(g)\)
Now we must go through the steps to get our final balanced equation:
Step 1: Split the equation into two half reactions. One oxidation half reaction and one reduction half reaction. A oxidation half reaction is when a species loses electrons, meaning the oxidation state increases. A reduction half reaction is when a species gains elecrtons, resulting in the decrease of the oxidation state.
\(\ce{C6H12O6}(aq)⟶\ce{6HCO3^-}(g)\) This is the oxidation half reaction. We must balance the equation before starting. We multiply the right side by 6 so their equal number of Carbon molecules. Carbon is losing electrons, going from an oxidation state of 0 to +24.
\(\ce{SO4^2-}(aq)⟶\ce{HS^-}(aq)\) This is the reduction half reaction because the sulfur is gaining electrons, going from an oxidation state of +6 to -2.
Step 2: Balance the oxygen by adding water:
\(\ce{C6H12O6}(aq)+\ce{12H2O}(l)⟶\ce{6HCO3^-}(g)\) We add 12 water molecules because the right side has 12 more oxygen than the left side.
\(\ce{SO4^2-}(aq)⟶\ce{HS^-}(aq)+\ce{4H2O}(l)\) Here we add 4 water molecules because the left side has 4 more oxygen than the right side.
Step 3: Balance hydrogen by adding protons(H+):
\(\ce{C6H12O6}(aq)+\ce{12H2O}(l)⟶\ce{6HCO3^-}(g)+\ce{30H^+}(aq)\) We need to add 30H+ to the right side to balance the hydrogen on the left side.
\(\ce{SO4^2-}(aq)+\ce{9H^+}(aq)⟶\ce{HS^-}(aq)+\ce{4H2O}(l)\) We need add 9H+ to the left side to balance the hydrogen.
Step 4: Balance the charge of each half reaction using electrons:
\(\ce{C6H12O6}(aq)+\ce{12H2O}(l)⟶\ce{6HCO3^-}(g)+\ce{30H^+}(aq)+\ce{24e^-}\) We must add 24 electrons on the right side to balance the charge. This is because Carbon loses 24electrons.
\(\ce{SO4^2-}(aq)+\ce{9H^+}(aq)+\ce{8e^-}⟶\ce{HS^-}(aq)+\ce{4H2O}(l)\) We must add 8 electrons to the left side of the equation to balance the charge. This is because Sulfur gains 8 electrons.
Step 5: Scale the reactions so that they have the same number of electrons:
\(\ce{3SO4^2-}(aq)+\ce{27H^+}(aq)⟶\ce{3HS^-}(aq)+\ce{12H2O}(l)\) To the reduction half reaction we must multiply by 3 in order to get 24 electrons.
We do not need to do anything to the oxidation half reaction.
Step 6: Add both half reactions and cancel substances on both sides. This is the final equation:
\(\ce{3SO4^2-}(aq)+\ce{C6H12O6}(aq)⟶\ce{6HCO3^-}(g)+\ce{3HS^-}(aq)+\ce{3H^+}\)
Q20.5.23
For the reduction of oxygen to water, E° = 1.23 V. What is the potential for this half-reaction at pH 7.00? What is the potential in a 0.85 M solution of NaOH?
S20.5.23
For the reduction of oxygen to water:
\(\ce{O2}(g)+\ce{H^+}(aq)+\ce{4e^-}⟶\ce{2H2O}(l)\)
Phase II: (additional comment) - Use the Nernst equation to solve this: E=E(standard)-(.0592V/2)log(Q)
For this reaction Q= 1/(pO2[H+]^4)
So, E= 1.23V-(.0592V/2)log(1/(pO2[H+]^4) ; Where p(O2)=.2 bar and H+= 1.0x10^-7
Therefore, E= 1.23V - .42= 0.81 V

