Extra Credit 4
- Page ID
- 82850
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)17.1.3
a) Fe^{3+} + 3e^- \to Fe
The definition of reduction is the gain of electrons. Since in this reaction the reactant is gaining electrons to become the product, reduction occurs.
b) Cr \to Cr^{3+} + 3e^-
The definition of oxidation is the loss of electrons. Since in this reaction the reactant is losing electrons, oxidation occurs.
c) MnO_4^{2-} \to MnO_4^- + e^-
The definition of oxidation is the loss of electrons. Since in this reaction the reactant is losing electrons, oxidation occurs.
d) Li^+ + e^- \to Li
The definition of reduction is the gain of electrons. Since in this reaction the reactant is gaining electrons to become the product, reduction occurs.
19.1.2
a) Ti: [Ar]4s^23d^2
Ti has 22 valence electrons. 18 of those are used to fill the base configuration of Ar, leaving 4 left. The 4s orbital has a lower energy to fill than the 3d orbital, so the 4s is filled first with two electrons. The remaining two electrons are distributed in the 3d orbital.
b) Ti^{2+}: [Ar]3d^2
Since Ti^{2+} loses two electrons from the 22 in Ti, there are 20 valence electrons in the ion. 18 are used to fill the Ar base configuration. While the 4s orbital has the lowest energy to fill, once the 4s and 3d orbitals have been filled with the given electrons, 4s becomes the orbital with the higher energy state. This means that electrons are lost first from the 4s orbital, then the 3d orbital.
c) Ti^{3+}: [Ar]3d^1
Taking the Ti^{2+} ion, in order to make the Ti^{3+} ion, an electron has to be lost. The 3d orbital is the only orbital outside the base of the Ar configuration, so the electron is lost from the 3d orbital, transitioning from 3d^2 to 3d^1.
d) Ti^{4+}: [Ar]
The last remaining 3d electron is the highest energy electron, so that is the one lost, giving the ion a configuration of Ar.
------
Phase 2: (Candice Zheng's take on the questions)
For these problems, I will be using the shorthand method of doing electron configurations.
I will go across each period to reach the element that I am interested in. For example, Li would be 1s2(2s1) because it goes through the first period and to reach Li on the periodic table, we would need to add another electron and it would be from the 2s shell. In another example, N would be 1s2(2s2)(2p3) because you need to go across the p shell to reach the N element. The d orbitals are included after Ca, so that Ca would be [Ar] 4s2 and Sc would be [Ar] 4s2(3d1). The order for adding electrons would be to go (from left to right) the shells:
s -> p -> d -> f
This is because generally, f requires more energy to fill than d and so on. The order for removing electrons would be to go in the same order as filling electrons because it would be easier to remove electrons from the s orbital than the p orbital.
1.Ti
The element Ti has no charge, so it is written just as like it is presented in the periodic table. The highest noble gas that is on a lower period for Ti would be Ar, so to simplify the electron configuration, we can start on Ar and build off from its electron configuration, since [Ar] = 1s2(2s2)2p6(3s2)3p6. So moving down the period, Ti would be [Ar]4s2(3d2) since we would need to add 2 electrons from the s orbital (with the energy level prefix 4) and 2 electrons from the d orbital (with the energy level prefix 3) to get to Ti.
2. Ti2+
This element has a charge of +2, so we would need the electron configuration of Ti without any charge and remove 2 electrons from it to have this positive charge. By taking what we already done in question one, Ti=[Ar]4s2(3d2), we can then remove 2 electrons by following the order s->p->d->f. We need to remove 2 electrons and we must remove all the s orbital electrons first before the d orbital electrons, so Ti2+ would be:
[Ar]3d2
3. Ti3+
This element has a charge of +3, so we would need the electron configuration of Ti without any charge and remove 3 electrons from it to have this positive charge. By taking what we already done in question one, Ti=[Ar]4s2(3d2), we can then remove 3 electrons by following the order s->p->d->f. We need to remove 2 electrons and we must remove all the s orbital electrons first before the d orbital electrons, so Ti3+ would be:
[Ar]3d1
since 2 electrons are removed from the 4s orbital and 1 electron is removed from the 3d orbital.
4. Ti4+
This element has a charge of +4, so we would need the electron configuration of Ti without any charge and remove 4 electrons from it to have this positive charge. By taking what we already done in question one, Ti=[Ar]4s2(3d2), we can then remove 4 electrons by following the order s->p->d->f. We need to remove 4 electrons and we must remove all the s orbital electrons first before the d orbital electrons, so Ti4+ would be:
[Ar]
since 2 electrons are removed from the 4s orbital and 2 electrons are removed from the 3d orbital.
------
19.2.4
a) [Pt(H_2O)_2Br_2] (square planar)

b) [Pt(NH_3)(py)(Cl)(Br)] (square planar)

c) [Zn(NH_3)_3Cl]^+ (tetrahedral)

d) [Pt(NH_3)_3Cl]^+ (square planar)

e) [Ni(H_2O)_4Cl_2]
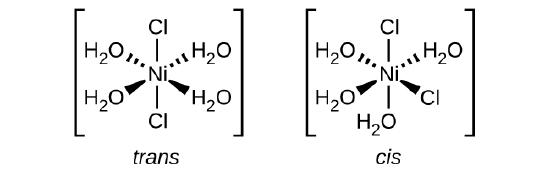
f) [Co(C_2O_4)_2Cl_2]^{-3}

------
Phase 2: (Candice Zheng's take on the questions)
Before we can start these problems, we have to define the different geometrical shapes that are mentioned in the problems.
-
Square planars have 4 bonding groups/domains on 'central' atom and 90 and 180 degree bond angles.
-
Tetrahedrals have 4 bonding groups/domains on 'central' atom and 109.5 degree bond angles.
-
Octahedrals have 6 bonding groups/domains on 'central' atom and 90 and 180 degree bond angles.
Each bidentate ion has 2 bites on the central metal atom.
Trans-isomers occur when the same atoms in a complex ion are 180 degrees from each other and Cis-isomers occur when the same atoms in a complex ion are 90 degrees from each other.
Optical isomers are two or more forms of a compound that have the same structure, but different optical activities because they are mirror images of each other. Optical isomers occur when there are no planes of symmetry on a complex ion.
1.[Pt(H2O)2Br2] (square planar)
There are 4 ions: 2 H2O and 2 Br. This means that since there are 2 ions of each and this complex is a square planar, so by using the definition of a square planar, the complex has both cis- and trans- isomers. If we place the 2 H2O so that they are 90 degrees from each other and the other ions on the other bonds, then we would get the answer on the left where the 2 H2O are cis with one another and the 2 Br are cis with one another. If we place the 2 H2O so that they are 180 degrees from each other (across) and the other ions on the other bonds, then we would get the answer on the right where the 2 H2O are trans with one another and the 2 Br are trans with one another. There are no optical isomers since there are planes of symmetry. There is a plane of symmetry that runs between the 2 H2O and the 2 Br (a straight horizontal line based off our solution) for the cis isomer. There is also a plane of symmetry that runs between the H2O and Br of the complex (so a straight horizontal line or a straight vertical line based off our solution).
2.[Pt(NH3)(py)(Cl)(Br)] (square planar, py = pyridine, C5H5N)
There are 4 ions: 1 NH3, 1 py, 1 Cl, and 1 Br. Since this is a square planar and all of these ions are different, then there are no cis-, trans-, or optical isomers (since there are planes of symmetry). The possible structures can be from rearranging the different ions while keeping one ion in the same spot. For the answer shown below, the NH3 was kept stationary whereas the other 3 ions were switched around, so that all the combinations were considered.
3.[Zn(NH3)3Cl]+ (tetrahedral)
There are 4 ions: 3 NH3 and 1 Cl. Since the complex is tetrahedral with 3 of its ions the same, there are no optical (since there are planes of symmetry), trans-, or cis- isomers. By using the definition of the tetrahedral (where there are only 109.5 degree angles), we can place the ions on any of the bonds. The complex has an overall +1 positive charge, so we need to put the complex in brackets and indicate the charge.
4. [Pt(NH3)3Cl]+ (square planar)
There are 4 ions: 3 NH3 and 1 Cl, but unlike question 3, this complex has a square planar shape, so we have to create both 180 degree and 90 degree angles. Since there are 3 of the same ions, there are no optical (since there are planes of symmetry), trans-, or cis- isomers. The complex has an overall +1 positive charge, so we need to put the complex in brackets and indicate the charge.
5. [Ni(H2O)4Cl2]
There are 6 ions, so this is an octahedral.
[Co(C2O4)2Cl2]3− (note that C2O2−4C2O42− is the bidentate oxalate ion, −O2CCO−2−O2CCO2−)
------
12.3.17
given H_2 + 2NO \to N_2O + H_2O
| [NO] (M) | 0.30 | 0.60 | 0.60 |
|---|---|---|---|
| [H2] (M) | 0.35 | 0.35 | 0.70 |
| Rate (mol/L/s) | 2.835 × 10−3 | 1.134 × 10−2 | 2.268 × 10−2 |
a) When the concentration of H_2 remains constant and the concentration of NO doubles, the rate increases from 2.835*10^{-3} to 1.134*10^{-2}. The ratio of 2:1 (the concentration of NO after and before) correlates to the ratio of (1.134*10^{-2})/(2.835*10^{-3}). The ratio (1.134*10^{-2})/(2.835*10^{-3}) is equivalent to 4:1. Hence, when the concentration of NO doubles, the rate increases by four, meaning that the order with respect to NO is 2. When the concentration of NO remains constant and the concentration of H_2 doubles, the rate goes from 1.134*10^{-2} to 2.268*10^{-2}. The ratio of 2:1 correlates to the ratio of (2.268*10^{-2}):(1.134*10^{-2}), which is equivalent to the ratio 2:1. Hence, since the rate doubles when H_2 doubles, the order with respect to H_2 is 1.
b) The rate equation of the reaction, using the orders of each of the reactants, is then rate = k[NO]^2[H_2], where k is the rate constant.
c) The rate equation and the table of values can be used to calculate the rate constant. 2.835*10^{-3} = k(0.3)^2(0.35) \Rightarrow k=0.09.
12.6.8
given 2NO + 2H_2 \to N_2 + 2H_2O
mechanism: 2NO + H_2 \to N_2 + H_2O_2 (slow)
H_2O_2 + H_2 \to 2H_2O (fast)
The slow step in a mechanism is the rate determining step. Since the slow step is the first step, and does not have any intermediates, then the rate law of the entire reaction is equivalent to the rate law of the first elementary, slow step. Hence, the rate law is rate = k[NO]^2[H_2]. The rate of an elementary reaction is the k constant times the molarity of the elements given. If there is a number in front of the element in the elementary reaction then in the rate law, the element is raised to the power of that number.
21.4.20
Find the age of a mummified primate that contains 8.25% of the original quantity of carbon 14.
The half life of carbon 14 is 5730 years. The equation relating half life, age, original quantity and current quantity is A = A_00.5^{t/t_{0.5}} where A is the current quantity, A_0 is the original quantity, t is the age, and t_{0.5} is the half life. This equation can be made into the equation: fraction left = 0.5^{t/t_{0.5}}. Using this equation and the data given can yield: 0.0825 = 0.5^{t/5730} \Rightarrow t=20 625 years.
20.3.8
Given NO_3^-(aq) + H^+(aq) + SO_3^{2-}(aq) \to SO_4^{-2}(aq) + HNO_2(aq)
a) reduction: 2e^- + 3H^+ + NO_3^- \to HNO_2 + H_2O
oxidation: H_2O +SO_3^{-2} \to SO_4^{-2} + 2H^+ +2e^-
Reduction occurs when electrons are gained in the forward reaction and oxidation occurs when electrons are lost in the forward reaction.
b) The anode is correlated to oxidation, so the anode corresponds to H_2O +SO_3^{-2} \to SO_4^{-2} + 2H^+ +2e^-. The cathode is correlated to reduction, so the cathode corresponds to 2e^- + 3H^+ + NO_3^- \to HNO_2 + H_2O.
c) Since in the oxidation reaction negatively charged electrons are released, the anode is positively charged. In the reduction reaction negatively charged electrons are added, so the cathode is negatively charged.
20.5.19
| Titrant (mL) | E (mV) |
|---|---|
| 2.00 | 50 |
| 6.00 | 100 |
| 9.00 | 255 |
| 10.00 | 960 |
| 11.00 | 1325 |
| 12.00 | 1625 |
| 14.00 | 1875 |
a) Ce^{4+} + Fe^{2+} \to Ce^{3+} + Fe^{3+} is the reaction for the oxidation of Fe^{2+} by Ce^{4+}; it is evident that Fe^{2+} is oxidized since the charge of the ion became more positive, indicating a loss of electrons.
b) By examining the graph of the plotted points below, the endpoint can be easily seen. The endpoint is the point at the graph where the rate of change of E is the greatest, which is at about the middle of the steepest section of the graph. Hence, the endpoint is at about 9 mL of the titrant added, or at around 600 mV.
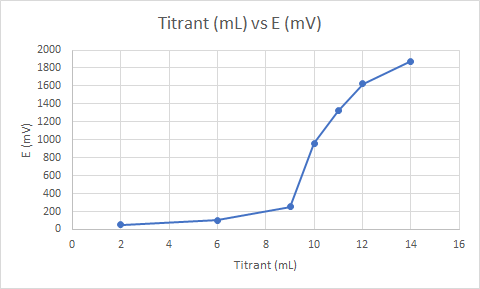
c) If the solution of Ce^{+4} was 0.1 M originally, and it took 9 mL of the 0.1 M solution to reach the endpoint, then around 0.9 millimoles of Ce^{+4} were used in the reaction. Since the oxidation of Fe^{2+} by Ce^{4+} has a 1:1 ratio of Ce^{4+} to Fe^{2+}, there hence follows that there were 0.9 millimoles of Fe^{2+} in the original solution.

