Extra Credit 37
- Page ID
- 82847
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Q17.5.5
An inventor proposes using a SHE (standard hydrogen electrode) in a new battery for smartphones that also removes toxic carbon monoxide from the air:
- Anode:
CO(g)+H2O(l)⟶CO2(g)+2H+(aq)+2e− CO(g)+H2O(l)⟶CO2(g)+2H+(aq)+2e−
with Eo(anode)=−0.53V Eo(anode)=−0.53V - Cathode:
2H+(aq)+2e−⟶H2(g) 2H+(aq)+2e−⟶H2(g)
with Eo(cathode)=0V Eo(cathode)=0V - Overall reaction:
CO(g)+H2O(l)⟶CO2(g)+H2(g) CO(g)+H2O(l)⟶CO2(g)+H2(g)
with Eocell=+0.53V Ecello=+0.53V
Would this make a good battery for smartphones? Why or why not?
Solution:
1) Recall the concept of spontaneity and connect spontaneity with electrochemistry

In this question, we only need to care about the relationship between Gibbs Free Energy and The Cell Potential
--> When the E-value is greater than zero, it indicates the spontaneity of a reaction, which will have high possibility to take place.
2) The overall cell potential is greater than zero, meaning the reaction is a spontaneous reaction.
Therefore, this might be a good battery for smartphones.
Q12.2.4
In the PhET Reactions & Rates interactive, on the Many Collisions tab, set up a simulation with 15 molecules of A and 10 molecules of BC. Select “Show Bonds” under Options.
1. Leave the Initial Temperature at the default setting. Observe the reaction. Is the rate of reaction fast or slow?
2. Click “Pause” and then “Reset All,” and then enter 15 molecules of A and 10 molecules of BC once again. Select “Show Bonds” under Options. This time, increase the initial temperature until, on the graph, the total average energy line is completely above the potential energy curve. Describe what happens to the reaction.
Solution:
1. Based on the observation from the simulation, the rate of reaction is quite slow.
2. When temperature increase, the kinetic energy will also increase, resulting the total average energy exceeds the potential energy. The equation below shows the relation between kinetic energy and temperature.
\[E_k=\frac{3}{2}kT\]
Ek = Kinetic Energy of Atom or Molecule in Joules
k = 1.38x10-23J
T = Temperature of Gas in Kelvin
Namely, the reactants will decrease and the products increase. Finally, there will have an approximately same amount of AB, BC and C and a little excess of A.
Q12.5.9
The rate constant at 325 °C for the decomposition reaction C4H8⟶2C2H4C4H8⟶2C2H4 is 6.1 × 10−8 s−1, and the activation energy is 261 kJ per mole of C4H8. Determine the frequency factor for the reaction.
Solution:
1) We need the Arrhenius equation to calculate the question.
\[k=Ae^\frac{-Ea}{RT}\]
k : Rate Constant
A : Frequency Factor or Pre-Exponential Factor
e : Mathematical Quantity
R : The Gas Consant
T : Kelvin Temperature
Ea : Activation Energy
2) To get the frequency factor, we need to re-write the Arrhenius equation as below
\[A=\frac{k}{e^\frac{-Ea}{RT}}\]
3) Plug in all the values, we get: (Be careful, we use R=8.314 J/mol*K, the activation energy = 261000 J)
\[A=\frac{6.1E-8}{e^\frac{-261000}{80314*(273+325)}}\]
,which equals the answer 3.9E15 s-1
Q21.4.4
Many nuclides with atomic numbers greater than 83 decay by processes such as electron emission. Explain the observation that the emissions from these unstable nuclides also normally include α particles.
Solution:
1) Think about the rule of conservation of a neutron to a proton:
n → p + e + ve
2) Then, think about the concept of stability:
The major reason for the alpha emission of α particles during a disintegration process of an element having atomic numbers bigger than 83 is that, if the α particle is emitted, the newly formed daughter nuclei will be closer to the band of stability.
Namely, the daughter nuclei will be more stable than the parent nuclei.
This explains the reason why the emissions from these unstable nuclides also normally include α particles.
Q20.2.8
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation.
- Mg(s) + Cu2+(aq) →
- Au(s) + Ag+(aq) →
- Cr(s) + Pb2+(aq) →
- K(s) + H2O(l) →
- Hg(l) + Pb2+(aq) →
Solution:
1) Recall the concept of a spontaneous reaction in the domain of electrochemistry:
Ecell = Ecathode - Eanode --> When Ecell is greater than 0, the reaction is spontaneous. If Ecell is smaller than 0, then, it is unlikely to see reaction undergo.
Link this concept to the activity series, we now know that the more reactivity the element has, the more likely it will be oxidized (lose electron(s))
2) According the activity series, find out the Ecell value for each reaction:
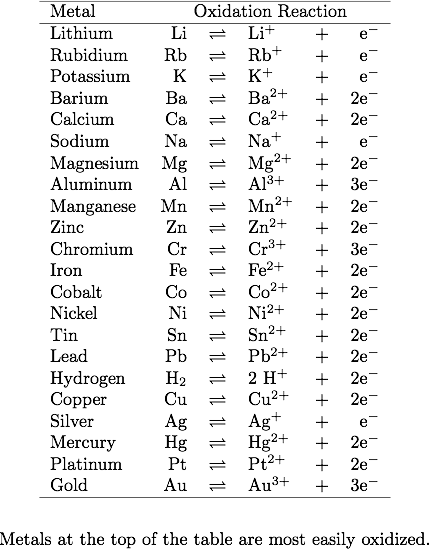
1. Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)
2. Less likely to happen
3. 2Cr(s) + 3Pb2+(aq) → 2Cr3++ 3Pb(s)
4. Unlike the previous three questions, this reaction is a replacement reaction.
Therefore, we can balance the reaction as 2K(s) + 2H2O(l) → H2(g)+ 2KOH(aq)
(P.S. If you check the position of oxygen from both side, you can still get the information of redox reaction that K has higher reactivity of oxygen compared to H2.)
5. Less likely to happen
Q20.5.3
In the equation wmax = −nFE°cell, which quantities are extensive properties and which are intensive properties?
Solution:
1) Know the definition of extensive properties and intensive properties first.
--> According to IUPAC, an intensive property is one whose magnitude is independent of the size of the system. An extensive property is one whose magnitude is additive for subsystems.
2) Examples of Intensive Properties:
Molality, Temperature, Pressure, Melting point and Boiling point, Color, etc.
3) Examples of Extensive Properties:
Mole, Entropy, Enthalpy, Mass, Volume
4) Apply concepts into the question:
--> We get E0cell as an intensive property and both n (mole) and Wmax as extensive properties.
Q20.9.12
Predict the products obtained at each electrode when aqueous solutions of the following are electrolyzed.
1. MgBr2 2. Hg(CH3CO2)2 3. Al2(SO4)3
Solution:
1) Recall the concept of the relation between electrode and redox reaction:
Cathode: Where reduction reaction take place VS Anode: Where oxidation reaction take place
2) General Guideline to solve this kind of question:
Electrolysis of aqueous solutions
→ Possible cathode half-reactions (reduction)
1. Reduction of H2O
2. Reduction of cations in the solution
→ Possible anode half-reactions (oxidation)
1. Oxidation of H2O
2. Oxidation of active metal electrodes
3. Oxidation of anions in the solution
→The half-reaction with the higher Eo value (having the stronger oxidizing agent) occurs on the cathode
→The half-reaction with the lower Eo value (having the stronger reducing agent) occurs on the anode
3) Apply the process from 2) to get the answer:
1. MgBr2
a) Cathode: 2H2O(aq) + 2e- → H2(g) + 2OH-
b) Anode: 2Br-(aq)→ Br2(l)+2e-
2. Hg(CH3CO2)2
a) Cathode: Hg2+(aq) + 2e- → Hg(l)
b) Anode: 2H2O (aq) → O2(g) + 4H+(aq) + 4e-
3. Al2(SO4)3
a) Cathode: 2H2O(aq) + 2e- → H2(g) + 2OH-
b) Anode: 2H2O (aq) → O2(g) + 4H+(aq) + 4e-
Q14.7.6
An area of intensive chemical research involves the development of homogeneous catalysts, even though homogeneous catalysts generally have a number of operational difficulties. Propose one or two reasons why a homogenous catalyst may be preferred.
Solution:
One reason for reference:
In homogeneous catalysis, the catalyst is in the same phase as the reactant(s). The number of collisions between reactants and catalyst is at a maximum because the catalyst is uniformly dispersed throughout the reaction mixture.
An illustration as the brief explanation for the differences between the homogeneous catalysis and the heterogeneous catalysis is attached below:


