Extra Credit 31
- Page ID
- 82841
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.4.4
Determine ΔG and ΔG° for each of the reactions in the previous problem.
a.) \(\ce{Hg}(l)+\ce{S^2-}(aq,\: 0.10\:M)+\ce{2Ag+}(aq,\: 0.25\:M)⟶\ce{2Ag}(s)+\ce{HgS}(s)\)
b.) The galvanic cell made from a half-cell consisting of an aluminum electrode in 0.015 M aluminum nitrate solution and a half-cell consisting of a nickel electrode in 0.25 M nickel(II) nitrate solution.
c.) The cell made of a half-cell in which 1.0 M aqueous bromine is oxidized to 0.11 M bromide ion and a half-cell in which aluminum ion at 0.023 M is reduced to aluminum metal. Assume the standard reduction potential for Br2(l) is the same as that of Br2(aq).
Q17.4.4
a.) First, write the half reactions of the given equation and find the corresponding values of Eº. Use the Standard Electrode (Half-Cell) Potentials Table.
\(cathode:\) \(2Ag^+(aq,0.25M)+2e^-⟶2Ag(s)\) \(E^\circ_{cathode}=0.7996 V\)
\(anode:\) \(Hg(l)+S^{2−}(aq,0.10M)⟶HgS(s)+2e^-\) \(E^\circ_{anode}=-0.70 V\)
The overall balanced chemical reaction is:
\(\ce{Hg}(l)+\ce{S^2-}(aq,\: 0.10\:M)+\ce{2Ag+}(aq,\: 0.25\:M)⟶\ce{2Ag}(s)+\ce{HgS}(s)\)
Now we need to calculate Eºcell. Recall \(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\) so:
\(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\)
\(E^\circ_{cell}=0.7996 V-(-0.70 V)\)
\(E^\circ_{cell}=1.4996 V\)
\(E^\circ_{cell}\approx 1.50 V\)
We want the answer rounded to two significant figures since the given molarity used two significant figures.
We can now calculate \(\Delta G^\circ\) using the equation \(\Delta G^\circ=-nFE^\circ_{cell}\) since we already found \(E^\circ_{cell}\) in the step above. We also know that n=2 because two electrons are transferred in the overall reaction. F is Faraday's constant.
\(\Delta G^\circ=-nFE^\circ_{cell}\)
\(\Delta G^\circ=−2mole×96.485\frac{kJ}{V·mol}×1.50 V\)
\(\Delta G^\circ=−289.46 kJ\)
Therefore \(\Delta G^\circ=-289.46 kJ\) for this reaction.
The reaction above is not under standard conditions so we will need to calculate \(E_{cell}\) first under the stated conditions before we calculate for \(\Delta G\). We can use the equation \(E_{cell}=E^\circ_{cell}-\frac{0.0592}{n}logQ\)
We already know the value for \(E^\circ\) and n from above. Q is the reaction quotient which is obtained from the overall balanced reaction equation above. Remember reaction quotients includes gaseous and aqueous states only. Solids and liquids has an activity of one and can be omitted in the quotient.
\(E_{cell}=E^\circ_{cell}-\frac{0.0592}{n}logQ\)
\(E_{cell}=1.50 V-\frac{0.0592}{2}log\frac{1}{[Ag^+]^2[S^{2-}]}\)
\(E_{cell}=1.50 V-\frac{0.0592}{2}log\frac{1}{(0.25)^2(0.10)}\)
\(E_{cell}=1.43 V\)
Now that we have found \(E_{cell}\) we can now find \(\Delta G\) for the stated conditions using the equation \(\Delta G=-nFE_{cell}\)
\(\Delta G=-nFE_{cell}\)
\(\Delta G=-2mole×96.485\frac{kJ}{V·mol}×1.43 V\)
\(\Delta G=-275.95 kJ\)
Therefore \(\Delta G=-275.95 kJ\) for this reaction.
b.) First, write out the half reactions based on the information given. After you write the half reactions, balance them, then you could tell which reaction is reduced and oxidized. For this reaction, aluminum electrode in 0.015 M aluminum nitrate is the anode and the nickel electrode in 0.25 M nickel(II) nitrate is the cathode. Anodes have electrons on the right and cathodes have electrons on the left.
\(cathode:\) \(Ni^{2+}(aq,0.25M)+2e^-⟶Ni(s)\) \(E^\circ_{cathode}=-0.257 V\)
\(anode:\) \(Al(s)⟶Al^{3+}(aq,0.015M)+3e^-\) \(E^\circ_{anode}=-1.662 V\)
When you balance the equations the number of electrons transferred is 6, so n=6.
Now we need to calculate Eºcell. Recall \(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\) so:
\(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\)
\(E^\circ_{cell}=-0.257 V-(-1.662 V)\)
\(E^\circ_{cell}=1.405 V\)
We can now calculate \(\Delta G^\circ\) using the equation \(\Delta G^\circ=-nFE^\circ_{cell}\) since we already found \(E^\circ_{cell}\) in the step above.
\(\Delta G^\circ=-nFE^\circ_{cell}\)
\(\Delta G^\circ=−6×96.485\frac{kJ}{V·mol}×1.405 V\)
\(\Delta G^\circ=−813.37 kJ\)
Therefore \(\Delta G^\circ=-813.37 kJ\) for this reaction.
The reaction above is not under standard conditions so we will need to calculate \(E_{cell}\) first under the stated conditions before we calculate for \(\Delta G\). We can use the equation \(E_{cell}=E^\circ_{cell}-\frac{0.0592}{n}logQ\)
We already know the value for \(E^\circ\) and n from above. Q is the reaction quotient which is obtained from the overall balanced reaction equation above. The Q for this reaction is \(\frac{[Al^{3+}]^2}{[Ni^{2+}]^3}\).
\(E_{cell}=E^\circ_{cell}-\frac{0.0592}{n}logQ\)
\(E_{cell}=1.405 V-\frac{0.0592}{6}log\frac{[Al^{3+}]^2}{[Ni^{2+}]^3}\)
\(E_{cell}=1.405 V-\frac{0.0592}{6}log\frac{(0.015)^2}{(0.25)^3}\)
\(E_{cell}=1.423 V\)
Now that we have found \(E_{cell}\) we can now find \(\Delta G\) for the stated conditions using the equation \(\Delta G=-nFE_{cell}\)
\(\Delta G=-nFE_{cell}\)
\(\Delta G=-6mole×96.485\frac{kJ}{V·mol}×1.423 V\)
\(\Delta G=-823.79 kJ\)
Therefore \(\Delta G=-823.79 kJ\) for this reaction.
c.) The cell made of a half-cell in which 1.0 M aqueous bromine is oxidized to 0.11 M bromide ion and a half-cell in which aluminum ion at 0.023 M is reduced to aluminum metal. For this problem, we are given that the bromine is getting oxidized and the aluminum ion is getting reduced. Bromine will be at the anode and aluminum will be at the cathode.
\(cathode:\) \(Al^{3+}(aq, 0.023M)+3e^- \rightarrow Al(s)\) \(E^\circ_{cathode}=-1.662 V\)
\(anode:\) \(2Br^-(aq, 0.11M) \rightarrow Br_2(aq, 1.0M)+2e^-\) \(E^\circ_{anode}=1.0872 V\)
When you balance the equations the number of electrons transferred is 6, so n=6.
Now we need to calculate Eºcell. Recall \(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\) so:
\(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\)
\(E^\circ_{cell}=-1.662 V-1.0872 V)\)
\(E^\circ_{cell}=-2.749 V\)
We can now calculate \(\Delta G^\circ\) using the equation \(\Delta G^\circ=-nFE^\circ_{cell}\) since we already found \(E^\circ_{cell}\) in the step above.
\(\Delta G^\circ=-nFE^\circ_{cell}\)
\(\Delta G^\circ=−6×96.485\frac{kJ}{V·mol}×-2.749 V\)
\(\Delta G^\circ=1591.4 kJ\)
Therefore \(\Delta G^\circ=1591.4 kJ\) for this reaction.
The reaction above is not under standard conditions so we will need to calculate \(E_{cell}\) first under the stated conditions before we calculate for \(\Delta G\). We can use the equation \(E_{cell}=E^\circ_{cell}-\frac{0.0592}{n}logQ\)
We already know the value for \(E^\circ\) and n from above. Q is the reaction quotient which is obtained from the overall balanced reaction equation above. The Q for this reaction is \(\frac{[Br_2]^3}{[Al^{3+}]^2[Br^-]^6}\).
\(E_{cell}=E^\circ_{cell}-\frac{0.0592}{n}logQ\)
\(E_{cell}=-2.749 V-\frac{0.0592}{6}log\frac{[Br_2]^3}{[Al^{3+}]^2[Br^-]^6}\)
\(E_{cell}=-2.749 V-\frac{0.0592}{6}log\frac{(1.0)^3}{(0.023)^2(0.11)^6}\)
\(E_{cell}=-2.838 V\)
Now that we have found \(E_{cell}\) we can now find \(\Delta G\) for the stated conditions using the equation \(\Delta G=-nFE_{cell}\)
\(\Delta G=-nFE_{cell}\)
\(\Delta G=-6mole×96.485\frac{kJ}{V·mol}×-2.838 V\)
\(\Delta G=1642.9 kJ\)
Therefore \(\Delta G=1642.9 kJ\) for this reaction.
Q12.1.4
A study of the rate of dimerization of C4H6 gave the data shown in the table:
\(\ce{2C4H6⟶C8H12}\)
| Time (s) | 0 | 1600 | 3200 | 4800 | 6200 |
|---|---|---|---|---|---|
| [C4H6] (M) |
1.00 x 10-2 |
5.04 x 10-3 |
3.37 x 10-3 |
2.53 x 10-3 |
2.08 x 10-3 |
- Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s.
- Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus [C4H6]. What are the units of this rate?
- Determine the average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b).
Q12.1.4
1.) The average rate of dimerization is the change in concentration of a reactant per unit time. In this case it would be:
\(rate\) \(of\) \(dimerization=-\frac{\Delta [C_4H_6]}{\Delta t}\)
Rate of dimerization between 0 s and 1600 s:
\(rate\) \(of\) \(dimerization=-\frac{5.04×10^{-3}M-1.00×10^{-2}M}{1600 s-0 s}\)
\(rate\) \(of\) \(dimerization=3.10 × 10^{-6} \frac{M}{s}\)
Rate of dimerization between 1600 s and 3200 s:
\(rate\) \(of\) \(dimerization=-\frac{3.37×10^{-3}M-5.04×10^{-3}M}{3200 s-1600 s}\)
\(rate\) \(of\) \(dimerization=1.04 × 10^{-6} \frac{M}{s}\)
2.) The instantaneous rate of dimerization at 3200 s can be found by graphing time versus [C4H6].
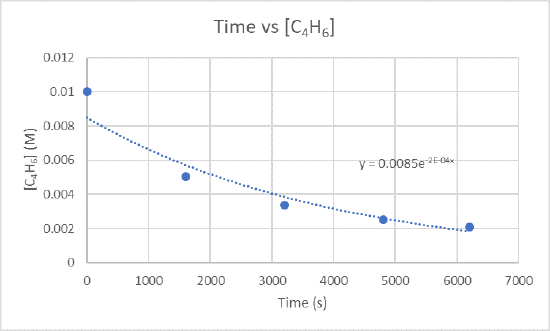
Because you want to find the rate of dimerization at 3200 s, you need to find the slope between 1600 s and 3200 s and also 3200 s and 4800 s.
For the slope between 1600 s and 3200 s use the points (1600 s, 5.04 x 10-3 M) and (3200 s, 3.37 x 10-3 M)
\(\frac{3.37×10^{-3}M-5.04×10^{-3}M}{3200 s-1600 s}\)
\(\frac{-0.00167 M}{1600 s}\)
\(-1.04×10^{-6}\frac{M}{s}\)
For the slope between 3200 s and 4800 s use the points (3200s, 3.37 x 10-3 M) and (4800s, 2.53 x 10-3 M)
\(\frac{2.53×10^{-3}M-3.37×10^{-3}M}{4800 s-3200 s}\)
\(\frac{-8.4×10^{-4} M}{1600 s}\)
\(-5.25×10^{-7}\frac{M}{s}\)
Take the two slopes you just found and find the average of them to get the instantaneous rate of dimerization.
\(\frac{-1.04×10^{-6}\frac{M}{s}+-5.25×x10^{-7}\frac{M}{s}}{2}\)
\(\frac{-1.565×10^{-6}\frac{M}{s}}{2}\)
\(-7.83×10^-7\frac{M}{s}\)
The instantaneous rate of dimerization is \(-7.83×10^-7\frac{M}{s}\) and the units of this rate is \(\frac{M}{s}\).
3.) The average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s can be found by using our answers from part a and b. If you look back up at the original equation, you could see that C4H6 and C8H12 are related in a two to one ratio. For every two moles of C4H6 used, there is one mole of C8H12 produced.
For this reaction, the average rate of dimerization and the average rate of formation can be linked through this equation:
\(\frac{-1}{2}\frac{\Delta [C_4H_6]}{\Delta t}=\frac{\Delta [C_8H_{12}]}{\Delta t}\)
Notice that reactant side is negative because the reactants are being used up in the reaction.
So, for the average rate of formation of C8H12 at 1600 s, use the rate of dimerization between 0 s and 1600 s we found earlier and plug into the equation:
\(\frac{-1}{2}×3.10 × 10^{-6} \frac{M}{s}=\frac{\Delta [C_8H_{12}]}{\Delta t}\)
\(\frac{\Delta [C_8H_{12}]}{\Delta t}=1.55×10^{-6}\frac{M}{s}\)
The average rate of formation for C8H12 at 1600 s is \(1.55×10^{-6}\frac{M}{s}\). The rate of formation will be positive because products are being formed.
The instantaneous rate of formation for C8H12 can be linked to the instantaneous rate of dimerization by this equation:
\(\frac{-1}{2}\frac{d[C_4H_6]}{dt}=\frac{d[C_8H_{12}]}{dt}\)
So, for the instantaneous rate of formation for C8H12 at 3200 s, use the value of instantaneous rate of dimerization at 3200 s found earlier and plug into the equation:
\(\frac{-1}{2}×-7.83×10^-7\frac{M}{s}=\frac{d[C_8H_{12}]}{dt}\)
\(\frac{d[C_8H_{12}]}{dt}=-3.92×10^{-7}\frac{M}{s}\)
The instantaneous rate of formation for C8H12 at 3200 s is \(-3.92×10^-7\frac{M}{s}\)
Q12.5.2
When every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs?
Q12.5.2
There has to be contact between reactants for a reaction to occur. The more the reactants collide, the more often reactions can occur. Factors that determine reaction rates include concentration of reactants, temperature, physical states of reactants, surface area, and the use of a catalyst. The reaction rate usually increases as the concentration of a reactant increases. Increasing the temperature increases the average kinetic energy of molecules, causing them to collide more frequently, which increases the reaction rate. When two reactants are in the same fluid phase, their particles collide more frequently, which increases the reaction rate. If the surface area of a reactant is increased, more particles are exposed to the other reactant therefore more collisions occur and the rate of reaction increases. A catalyst participates in a chemical reaction and increases the reaction rate without changing itself.
Q21.3.6
Technetium-99 is prepared from 98Mo. Molybdenum-98 combines with a neutron to give molybdenum-99, an unstable isotope that emits a β particle to yield an excited form of technetium-99, represented as 99Tc*. This excited nucleus relaxes to the ground state, represented as 99Tc, by emitting a γ ray. The ground state of 99Tc then emits a β particle. Write the equations for each of these nuclear reactions.
Q21.3.6
There are a total of five equations we need to write.
The first equation is for this nuclear reaction: Technetium-99 is prepared from 98Mo
Technetium has the atomic number 43 and Molybdenum is 42. This equation is the most simple since it doesn't have an alpha or beta particles being emitted.
\({^{98}_{42}Mo}\rightarrow{^{99}_{43}Tc}\)
The second equation is for: Molybdenum-98 combines with a neutron to give molybdenum-99
Recall that a neutron is \({^{1}_{0}n}\)
Always check to make sure the sum of the mass numbers of the reactants equals the sum of the mass numbers of the products and the sum of the charges of the reactants equals the sum of the charges of the products.
\({^{98}_{42}Mo}+{^{1}_{0}n}\rightarrow{^{99}_{42}Mo}\)
\(mass\) \(number:\) \(98+1=99\)
\(charge: 42+0=42\)
The third equation is for: Molybdenum-99, an unstable isotope emits a β particle to yield an excited form of technetium-99, represented as 99Tc*
Recall a β particle is \({^{0}_{-1}β}\)
The unstable isotope emits a β particle and yields 99Tc*, so the β particle and 99Tc* will be on the product side.
\({^{99}_{42}Mo}\rightarrow{^{99}_{43}Tc^*}+{^{0}_{-1}β}\)
Again, make sure the mass numbers and charges are balanced on both sides.
\(mass\) \(number:\) \(99=0+99\)
\(charge: 42=43+-1\)
The fourth equation is for: 99Tc*,t his excited nucleus relaxes to the ground state, represented as 99Tc, by emitting a γ ray
Recall a γ ray is \({^{0}_{0}γ}\)
γ ray is being emitted so it will be on the product side. Check mass numbers and charges to make sure they are balanced on both the reactant and product side.
\({^{99}_{43}Tc^*}\rightarrow{^{99}_{43}Tc}+{^{0}_{0}γ}\)
\(mass\) \(number:\) \(99=0+99\)
\(charge: 43=43+0\)
The fifth and final equation is for: The ground state of 99Tc then emits a β particle
β particle is being emitted so it will be on the product side.
\({^{99}_{43}Tc}\rightarrow{^{99}_{44}Ru}+{^{0}_{-1}β}\)
Check charges and mass numbers to make sure they are balanced on both sides.
\(mass\) \(number:\) \(99=0+99\)
\(charge: 43=44+-1\)
Q20.2.2
If two compounds are mixed, one containing an element that is a poor oxidant and one with an element that is a poor reductant, do you expect a redox reaction to occur? Explain your answer. What do you predict if one is a strong oxidant and the other is a weak reductant? Why?
Q20.2.2
Note: Species in the standard reduction potentials table that lie below H2 are stronger reductants (more easily oxidized) than H2. Species that lie above H2 are stronger oxidants (more easily reduced). The reductant is the substance that loses electrons and is oxidized in the process; the oxidant is the species that gains electrons and is reduced in the process.
If two compounds are mixed, one element is a poor oxidant and one is a poor reductant, a redox reaction will not likely occur. The poor oxidant will not be strong enough reduce and the poor reductant will also not be strong enough to oxidize.
When a strong oxidant and a weak reductant are mixed, a redox reaction is likely to occur. The strong oxidant will be strong enough to reduce. Even though, there is a weak reductant, the weak reductant will still be able to oxidize because if a reduction occurred from the strong oxidant, an oxidation will also occur by the weak reductant. A reduction must always be accompanied by an oxidation and vice versa.
Q20.4.21
Each reaction takes place in acidic solution. Balance each reaction and then determine whether it occurs spontaneously as written under standard conditions.
a. \(Se(s) + Br_2(l) → H_2SeO_3(aq) + Br^−(aq)\)
b. \(NO_3^−(aq) + S(s) → HNO_2(aq) + H_2SO_3(aq)\)
c. \(Fe^3{^+}(aq) + Cr^3{^+}(aq) → Fe^2{^+}(aq) + Cr_2O_7^2{^−}(aq)\)
Q20.4.21
a.) \(Se(s)+Br_2(l)→H_2SeO_3(aq)+Br^−(aq)\)
Start out by writing out both the half reactions.
\(Br_2(l)\rightarrow Br^-(aq)\)
\(Se(s)\rightarrow H_2SeO_3(aq)\)
The oxidation state of bromine on the left side is 0. The oxidation state of bromine on the right is -1. Because the oxidation state of bromine decreases from 0 to -1, this is the reduction half-reaction (cathode). That means the selenium half reaction is the oxidation half reaction (anode).
Now we can balance both the half reactions.
\(cathode:\) \(Br_2(l)\rightarrow Br^-(aq)\)
\(anode:\) \(Se(s)\rightarrow H_2SeO_3(aq)\)
1. Balance all the elements in the equations except O and H.
\(Br_2(l)\rightarrow 2Br^-(aq)\)
\(Se(s)\rightarrow H_2SeO_3(aq)\)
2. Balance the oxygen atoms by adding H2O molecules to the opposite side of the equation.
\(Br_2(l)\rightarrow 2Br^-(aq)\)
\(3H_2O(l)+Se(s)\rightarrow H_2SeO_3(aq)\)
3. Balance the hydrogen atoms by adding H+ ions to the opposite side of the equation.
\(Br_2(l)\rightarrow 2Br^-(aq)\)
\(3H_2O(l)+Se(s)\rightarrow H_2SeO_3(aq)+4H^+(aq)\)
4. Add up the charges on each side. Add electrons (e-) to the more positive side if needed.
\(2e^-+Br_2(l)\rightarrow 2Br^-(aq)\)
\(3H_2O(l)+Se(s)\rightarrow H_2SeO_3(aq)+4H^+(aq)+4e^-\)
5. The electrons on each side must be made equal. If they are not, they need to be multiplied the lowest common multiple to be made the same.
\(2(2e^-+Br_2(l)\rightarrow 2Br^-(aq))\)
\(4e^-+2Br_2(l)\rightarrow 4Br^-(aq)\)
\(3H_2O(l)+Se(s)\rightarrow H_2SeO_3(aq)+4H^+(aq)+4e^-\)
6. Add the half reactions equations together. Common terms should be canceled out.
\(3H_2O(l)+Se(s)+2Br_2(l)\rightarrow H_2SeO_3(aq)+4H^+(aq)+4Br^-(aq)\)
Now we need to figure out if the reaction occurs spontaneously as written under standard conditions. We need to calculate the standard cell potential (\(E^\circ_{cell}\)). If \(E^\circ_{cell}\) is positive (\(E^\circ_{cell}\)>0) then the reaction is spontaneous. If \(E^\circ_{cell}\) is negative (\(E^\circ_{cell}\)<0) then the reaction is not spontaneous. We already determined which reactions occurred in the anode and cathode above. Now we need to find the standard reduction potentials of the anode and cathode using the standard reduction potentials chart.
\(cathode:\) \(2e^-+Br_2(l)\rightarrow 2Br^-(aq)\) \(E^\circ_{cathode}=1.066 V\)
\(anode:\) \(3H_2O(l)+Se(s)\rightarrow H_2SeO_3(aq)+4H^+(aq)+4e^-\) \(E^\circ_{anode}=0.74 V\)
Now we can calculate \(E^\circ_{cell}\)
\(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\)
\(E^\circ_{cell}=1.066 V-0.74 V\)
\(E^\circ_{cell}=0.33 V\)
\(E^\circ_{cell}\) is greater than zero, so the reaction is spontaneous.
b.) \(NO^−_3(aq)+S(s)→HNO_2(aq)+H_2SO_3(aq)\)
Start out by writing out both the half reactions.
\(NO^-_3(aq)\rightarrow HNO_2(aq)\)
\(S(s)\rightarrow H_2SO_3(aq)\)
Balance both the half reactions.
1. Balance all the elements in the equations except O and H.
\(NO^-_3(aq)\rightarrow HNO_2(aq)\)
\(S(s)\rightarrow H_2SO_3(aq)\)
2. Balance the oxygen atoms by adding H2O molecules to the opposite side of the equation.
\(NO^-_3(aq)\rightarrow HNO_2(aq)+H_2O(l)\)
\(3H_2O(l)+S(s)\rightarrow H_2SO_3(aq)\)
3. Balance the hydrogen atoms by adding H+ ions to the opposite side of the equation.
\(3H^+(aq)+NO^-_3(aq)\rightarrow HNO_2(aq)+H_2O(l)\)
\(3H_2O(l)+S(s)\rightarrow H_2SO_3(aq)+4H^+(aq)\)
4. Add up the charges on each side. Add electrons (e-) to the more positive side if needed.
\(2e^-+3H^+(aq)+NO^-_3(aq)\rightarrow HNO_2(aq)+H_2O(l)\)
\(3H_2O(l)+S(s)\rightarrow H_2SO_3(aq)+4H^+(aq)+4e^-\)
5. The electrons on each side must be made equal. If they are not, they need to be multiplied the lowest common multiple to be made the same.
\(2(2e^-+3H^+(aq)+NO^-_3(aq)\rightarrow HNO_2(aq)+H_2O(l))\)
\(4e^-+6H^+(aq)+2NO^-_3(aq)\rightarrow 2HNO_2(aq)+2H_2O(l)\)
\(3H_2O(l)+S(s)\rightarrow H_2SO_3(aq)+4H^+(aq)+4e^-\)
6. Add the half reactions equations together. Common terms should be canceled out.
\(2NO^-_3(aq)+H_2O(l)+2H^+(aq)+S(s)\rightarrow H_2SO_3(aq)+2HNO_2(aq)\)
Now we need to figure out if the reaction occurs spontaneously as written under standard conditions. We need to calculate the standard cell potential (\(E^\circ_{cell}\)). If \(E^\circ_{cell}\) is positive (\(E^\circ_{cell}\)>0) then the reaction is spontaneous. If \(E^\circ_{cell}\) is negative (\(E^\circ_{cell}\)<0) then the reaction is not spontaneous. Cathodes will usually have electrons on the left side of the equation and anodes will usually have electrons on the right side of the equation. Use the standard reduction potentials chart to find the corresponding potentials.
\(cathode:\) \(2e^-+3H^+(aq)+NO^-_3(aq)\rightarrow HNO_2(aq)+H_2O(l)\) \(E^\circ_{cathode}=0.934 V\)
\(anode:\) \(3H_2O(l)+S(s)\rightarrow H_2SO_3(aq)+4H^+(aq)+4e^-\) \(E^\circ_{anode}=0.449 V\)
Now we can calculate \(E^\circ_{cell}\)
\(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\)
\(E^\circ_{cell}=0.934 V-0.449 V\)
\(E^\circ_{cell}=0.485 V\)
\(E^\circ_{cell}\) is greater than zero, so the reaction is spontaneous.
c.) \(Fe^{3+}(aq)+Cr^{3+}(aq)→Fe^{2+}(aq)+Cr_2O^{2−}_7(aq)\)
Start out by writing out both the half reactions.
\(Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\)
\(Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)\)
The oxidation state of iron on the left side is +3. The oxidation state of iron on the right is +2. Because the oxidation state of iron decreases from +3 to +2, this is the reduction half-reaction (cathode). That means the chromium half reaction is the oxidation half reaction (anode).
Now we can balance both the half reactions.
\(cathode:\) \(Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\)
\(anode:\) \(Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)\)
1. Balance all the elements in the equations except O and H.
\(Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\)
\(2Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)\)
2. Balance the oxygen atoms by adding H2O molecules to the opposite side of the equation.
\(Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\)
\(7H_2O(l)+2Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)\)
3. Balance the hydrogen atoms by adding H+ ions to the opposite side of the equation.
\(Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\)
\(7H_2O(l)+2Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)+14H^+(aq)\)
4. Add up the charges on each side. Add electrons (e-) to the more positive side if needed.
\(e^-+Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\)
\(7H_2O(l)+2Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)+14H^+(aq)+6e^-\)
5. The electrons on each side must be made equal. If they are not, they need to be multiplied the lowest common multiple to be made the same.
\(6(e^-+Fe^{3+}(aq)\rightarrow Fe^{2+}(aq))\)
\(6e^-+6Fe^{3+}(aq)\rightarrow 6Fe^{2+}(aq)\)
\(7H_2O(l)+2Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)+14H^+(aq)+6e^-\)
6. Add the half reactions equations together. Common terms should be canceled out.
\(7H_2O(l)+2Cr^{3+}(aq)+6Fe^{3+}(aq)\rightarrow 6Fe^{2+}(aq)+Cr_2O^{2-}_7(aq)+14H^+(aq)\)
Now we need to figure out if the reaction occurs spontaneously as written under standard conditions. We need to calculate the standard cell potential (\(E^\circ_{cell}\)). If \(E^\circ_{cell}\) is positive (\(E^\circ_{cell}\)>0) then the reaction is spontaneous. If \(E^\circ_{cell}\) is negative (\(E^\circ_{cell}\)<0) then the reaction is not spontaneous. We already determined which reactions occurred in the anode and cathode above. Now we need to find the standard reduction potentials of the anode and cathode using the standard reduction potentials chart.
\(cathode:\) \(e^-+Fe^{3+}(aq)\rightarrow Fe^{2+}(aq)\) \(E^\circ_{cathode}=0.771 V\)
\(anode:\) \(7H_2O(l)+2Cr^{3+}(aq)\rightarrow Cr_2O^{2-}_7(aq)+14H^+(aq)+6e^-\) \(E^\circ_{anode}=1.36 V\)
Now we can calculate \(E^\circ_{cell}\)
\(E^\circ_{cell}=E^\circ_{cathode}-E^\circ_{anode}\)
\(E^\circ_{cell}=0.771 V-1.36 V\)
\(E^\circ_{cell}=-0.59 V\)
\(E^\circ_{cell}\) is less than zero, so the reaction is not spontaneous.
Q20.9.6
Electrolysis is the most direct way of recovering a metal from its ores. However, the Na+(aq)/Na(s), Mg2+(aq)/Mg(s), and Al3+(aq)/Al(s) couples all have standard electrode potentials (E°) more negative than the reduction potential of water at pH 7.0 (−0.42 V), indicating that these metals can never be obtained by electrolysis of aqueous solutions of their salts. Why? What reaction would occur instead?
Q20.9.6
Electrolysis is the use of an electric current to stimulate a non-spontaneous reaction. Electrolysis can be used to separate a substance into its original components/elements. Na+(aq)/Na(s), Mg2+(aq)/Mg(s) and Al3+(aq)/Al(s) have more negative standard electrode potentials than the standard electrode potential of water. Remember species in the standard reduction potentials table that lie below H2 are stronger reductants they are more easily oxidized. For electrolysis to take place, the elements must have higher standard electrode potential than water, so it can be oxidized. These elements are lower on the standard reduction potentials table than water which shows that they will not react with water, electrolysis will not occur. No reaction will occur because the elements are already in its oxidized state, it cannot be oxidized even further.
Q14.5.2
For any given reaction, what is the relationship between the activation energy and each of the following?
- electrostatic repulsions
- bond formation in the activated complex
- the nature of the activated complex
S14.5.2
1.) Electrostatic repulsion: Electrostatic repulsion is the unfavorable interaction between two species of like charge. Activation energy is the minimum amount of energy needed for a reaction to occur. Reacting molecules must have enough energy to overcome electrostatic repulsion, and a minimum amount of energy is required to break chemical bonds so that new ones may be formed.
2.) Bond formation in the activated complex: An activated complex is an intermediate state that is formed during the conversion of reactants into products. Bond breaking can increase activation energy as breaking bonds requires energy.
3.) Nature of the activated complex: The activation energy of a chemical reaction is the difference between the energy of the activated complex and the energy of the reactants. If the structure has a high steric hindrance the activation energy will be higher.

