Extra Credit 29
- Page ID
- 82838
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.4.2
For the ΔG° values here, determine the standard cell potential for the cell.
a. 12 kJ/mol, n=3
b. -45 kJ/mol, n=1
S17.4.2
a. We are given ΔG° (Gibb's Free Energy under standard conditions), and the number of moles. We are being asked to find Standard Cell Potential, and so we can use the following equation:
Solving for E°,
.
We know that ΔG°=12kJ/mol, n=3, and F is Faraday's constant, which is 96,485 C/mol.
However, cell potential is expressed in terms of volts, and this equation gives an answer in terms of kJ/C. We must convert from kJ to J, which gives units of J/C. Because 1 volt = 1J/C, our answer will be in the proper units.
This answer makes sense because we started with a positive ΔG° value and got a negative E° value, which both indicate that the reaction is not spontaneous. E° and ΔG° values for a reaction should always have opposite signs.
E°<0 not spontaneous
E°>0 spontaneous
b. Solving this problem the same way,
.
Again, this solution makes sense because the reaction had a negative ΔG° value, indicating that it is spontaneous, and the positive E° value confirms this.
Q12.1.2
Ozone decomposes to oxygen according to the equation 2 O3 (g) ---> 3 O2 (g). Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen.
S12.1.2
For the general reaction, aA ---> bB, the rate of the reaction can be expressed in terms of the disappearance of A or the appearance of B over a certain time period as follows.
$$- \dfrac{1}{a}\dfrac{\Delta [A]}{\Delta t} = - \dfrac{1}{b}\dfrac{\Delta [B]}{\Delta t} = \dfrac{1}{c}\dfrac{\Delta [C]}{\Delta t} = \dfrac{1}{d}\dfrac{\Delta [D]}{\Delta t}$$
We want the rate of a reaction to be positive, but the change in the concentration of a reactant, A, will be negative because it is being used up to be transformed into product, B. Therefore, when expressing the rate of the reaction in terms of the change in the concentration of A, it is important to add a negative sign in front to ensure the overall rate positive.
Lastly, the rate must be normalized according to the stoichiometry of the reaction. In the decomposition of ozone to oxygen, two moles of ozone form three moles of oxygen gas. This means that the increase in oxygen gas will be 1.5 times as great as the decrease in ozone. Because the rate of the reaction should be able to describe both species, we divide the change in concentration by its stoichiometric coefficient in the balanced reaction equation to deal with this issue.
Therefore, the rate of the reaction of the decomposition of ozone into oxygen gas can be described as follows:
$$Rate=-\frac{Δ[O3]}{2ΔT}=\frac{Δ[O2]}{3ΔT}$$
Q12.4.20
![]()
For the past 10 years, the unsaturated hydrocarbon 1,3-butadiene (CH2=CH–CH=CH2)(CH2=CH–CH=CH2) has ranked 38th among the top 50 industrial chemicals. It is used primarily for the manufacture of synthetic rubber. An isomer exists also as cyclobutene:
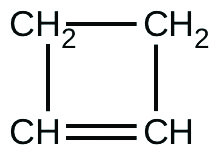
The isomerization of cyclobutene to butadiene is first-order and the rate constant has been measured as 2.0 × 10−4 s−1 at 150°C in a 0.53-L flask. Determine the partial pressure of cyclobutene and its concentration after 30.0 minutes if an isomerization reaction is carried out at 150 °C with an initial pressure of 55 torr.
S12.4.20
We are looking at the reaction 1,3-butadiene ---> cyclobutene. We know that the reaction is first-order, and that its rate constant, k, is 2.0x10-4/sec at the given conditions. Therefore, the rate can be described as
$$Rate=k[cyclobutene]=2.0\ x\ 10^{-4}sec^{-1}[cyclobutene]$$
For first order reactions, the concentration of cyclobutene at any time of the reaction can be described from the integrated form of the rate law.
$$ln[cyclobutene]_{t}=-k\ x\ t\ +[cyclobutene]_0$$
We must start by determining the initial concentration of cyclobutene. Concentration is expressed in moles per liter. We know that the volume of this system is 0.53L, but we do not know the number of moles. However, we can use the ideal gas equation because we know pressure (55 torr), volume (0.53 L), R (universal gas constant, 0.08206L*atm/mol*K), and temperature (150°C).
$$PV=nRT$$
$$n=\frac{PV}{RT}=\frac{55\ torr\ x\ 0.53L}{0.08206 \frac{Lxatm}{molxK} x 423.15K}$$
Thus, n=0.00110 moles of cyclobutene, and the initial concentration of cyclobutene is
$$[cyclobutene]_0=\frac {n}{V}=\frac{0.00110mol}{0.53L}=0.00208M$$
Going back to the integrated rate law and substituting 2.0x10-4 sec-1 for k, 30 minutes for t, and 0.00208M for [cyclobutene]0
$$ln[cyclobutene]_{30min}=\frac{-2.0x10^{-4}}{sec} x 30min + ln[0.00208]$$
Converting from minutes to seconds and simplifying the natural logarithm,
$$ln[cyclobutene]_{30min}=\frac{-2.0x10^{-4}}{sec} x 30min x \frac{60sec}{1min}-6.173=-6.533$$
$$[cyclobutene]_{30min}=e^{-6.533}=0.00145M$$
Going back to the ideal gas equation to determine the the partial pressure,
$$P=\frac{n}{v}xRT=0.00145\frac{mol}{L}x0.08206\frac{Lxatm}{molxK}x(150+273)K$$
$$P=0.0505atmx\frac{760torr}{1 atm}=38 torr$$
The concentration of the cyclobutene after 30 minutes will be 0.0015M, and the partial pressure will be 38 torr.
Q21.3.4
Complete each of the following equations:
1.
2.
3.
4.
S21.3.4
Nuclear nomenclature is denoted in terms of A and Z, where A is the mass number and Z is atomic number, which is also the number of protons.
Because the mass number, A, is the total number of protons and neutrons, the number of neutrons can be found by subtracting the number of protons, Z, from A.
# of neutrons = Mass number - # of protons = A - Z.
Radioctivity involves alpha, beta and gamma ray particles:
Alpha - \(\ce{^{4}_{2}He}\)
Beta- \(\ce{^{0}_{-1}\beta ^-}\) or \(\ce{^{0}_{1}\beta ^+}\)
Gamma- \(\gamma\)
1.
On the reactants side of the equation, we have a lithium-7 atom reacting with something to form 2 alpha particles. The lithium-7 atom has 3 protons and a mass number of 7. The two helium atoms have a total of 4 protons and a total mass of 8, so whatever reacts with helium must have 1 proton and a mass of 1. A proton has there properties, so the reaction is:
2.
In this reaction, carbon-14 is emitting something to form nitrogen-17. The mass number, 14, is conserved when carbon-14 is decayed into nitrogen-14. The change is in the number of protons, as carbon has 6 protons and nitrogen has 7 protons. Therefore, the emitted particle must have a charge of -1 and a mass of 0. This is a case of ß- decay, as a neutron is being converted into an proton by emitting an electron.
or
3.
This is a nuclear transmutation reaction, where an aluminum-27 atom is reacting with a beta particle to form something and emit a neutron. There are a total of 15 protons and a mass number of 31 on the reactants side. The neutron has no charge and no protons, but a mass of 1. Therefore, the reaction between the aluminum and alpha particle must produce, along with the neutron, something with a mass number of 30 and 15 protons, which is a phosphorus-30 atom. Thus, the reaction is
4.
In this nuclear decay reaction, Curium-250 emits 2 neutrons and forms something and Strontium-98. The Curium-250 has a mass number of 250 and 96 protons. The Strontium-98 and four neutrons together have a mass number of 102 and 38 protons. Therefore, the other product must have a mass number of 148(250-102) and a total of 58(96-38) protons, which is Cerium-148.
Q20.1.1
Identify the oxidation state of the atoms in the following compounds:
- PCl3
- CO32-
- H2S
- S8
- SCl2
- Na2SO3
- SO42−
S20.1.1
A few general notes for determining oxidation states:
The more electronegative element will have a negative oxidation state because it will attract the electrons more, and the thus have a more negative charge. The sum of the oxidation numbers in a compound equals 0, and the sum of the oxidation numbers in a polyatomic ion is its charge.
1. PCl3: Chlorine is a Group 7A element, and Group 7A elements tend to have 1- oxidation numbers, as they require one electron to form noble gas configuration. Therefore, the chlorine atoms in PCl3 will have an oxidation state of 1-. Because there are three chlorine atoms, 0 = ox. # of phosphorus + 3(-1), so the ox. # of phosphorus is 3+.
\(3(-1)+3=0\)
P: 3+ Cl: 1-
2. CO32-: This is the carbonate ion, so the sum of the oxidation states of the constituent atoms must sum to -2. There are three oxygen atoms which will take their normal oxidation state of 2-. Therefore, -2= (oxidation state of C) + 3(-2). Thus, the oxidation state of the carbon atom is -2 + 6 = 4.
C: 4+ O: 2-
3. H2S: When bonded to nonmetals, hydrogen takes an oxidation state of 1+. Therefore, 0=2(+1) + ox. # of sulfur, so the oxidation number of sulfur is 2-.
H: 1+ S: 2-
4. S8: S8 is composed of only sulfur atoms which all have the same electronegativity, and they will all share the electrons equally. This is the elemental from of sulfur. Therefore, the oxidation number on the sulfur atoms is 0.
S: 0
5. SCl2: Chlorine is a Group 7A element, and thus will have a 1- oxidation state. Therefore, the oxidation number of the sulfur atom will have to be 2+, as there are two chlorine atoms (0=ox. # of sulfur + 2(-1)).
S: 2+ Cl:1-
6. Na2SO3: This is an ionic compound consisting of two sodium ions bonded to a sulfite ion. The oxidation numbers of the sodium atoms will be equal to their ionic charge, which is 1+, as they in Group 1A, and lose 1 electron to attain noble gas configuration. The overall charge of the sulfite ion is 2-, which is equal and opposite to the overall charge of the two sodium ions. Within the sulfite ion, the oxygen atoms take a 2- oxidation number. This means that -2=ox. # of sulfur atom + 3(-2), and thus, the ox. # of the sulfur atom is 4+.
Na: 1+ S:4+ O: 2-
We can check this by making sure the sum of all of the oxidation numbers equals to 0: 2(+1) + 1(+4) + 3(-2)=0, so we are correct.
7. SO42-: This is the sulfate ion, which has a charge of 2-, so the sum of the oxidation numbers must equal to -2. The oxygen atoms will have an oxidation number of 2-. Therefore, the oxidation number of the sulfur atom can be found from -2 = ox. # of sulfur + 4(-2), which gives 6+.
S: 6+ O:2-
Q20.4.19
![]()
Carbon is used to reduce iron ore to metallic iron. The overall reaction is as follows:
2Fe2O3•xH2O(s) + 3C(s) → 4Fe(l) + 3CO2(g) + 2xH2O(g)
Write the two half-reactions for this overall reaction.
S20.4.19
We must start by determining the oxidation numbers of all of the atoms in the reaction to determine which species is being reduced and which is being oxidized.
Starting with Fe2O3, we know that oxygen has a 2- charge when bonded with metals, so its oxidation number is 2-. Because there are 3 oxygen atoms and 2 iron atoms, the iron must have a 3+ oxidation number, as 2(3)+3(-2)=0.
The water is conserved on both sides of the equation, so the oxidation states of the hydrogen and oxygen will remain the same, 1+ and 2- respectively.
Carbon(s) is not bonded to anything else, so its oxidation number as 0. Likewise, the iron on the products side of the reaction is elemental, and also has an oxidation number of 0.
Lastly, we must determine the oxidation numbers for the elements in carbon dioxide. The oxygen atoms will have oxidation numbers of 2-. Then, the oxidation number on the carbon can be found as follows: 0=ox.# of C + 2(-2), so ox.# of C=4.
2Fe2O3•xH2O(s) + 3C(s) → 4Fe(l) + 3CO2(g) + 2xH2O(g)
ox#: 3+ 2- 1+ 2- 0 0 4+ 2- 1+ 2-
By looking at the oxidation numbers, we can see that iron's oxidation number is reduced from 3+ to 0, meaning that each iron atom gained 3 electrons. Carbon's oxidation number increased from 0 to 4+, meaning that each carbon atom gained 4 electrons.
Now, we can split up the reactions into the reduction and oxidation half reactions. Because iron is gaining electrons and thus being reduced, the reduction reaction is iron(III) oxide turning into iron metal. The oxidation reaction is carbon(s) turning into carbon dioxide.
$$Reduction: 2Fe_{2}O_{3}\cdot xH_{2}O(s)\rightarrow4Fe(l)+2xH_{2}O(g)$$
$$Oxidation: 3C(s)\rightarrow3CO_{2}(g)$$
Now, we must balance each reaction. Starting with the reduction reaction, we start by balancing the iron atoms. There are 2 iron atoms in iron(III) oxide, so the products should have 2 iron atoms.
Fe2O3•xH2O(s) ---> 2Fe(l)
$$Fe_{2}O_{3}\cdot xH_{2}O(s)\rightarrow2Fe(l))$$
Next, we balance the oxygen by adding water molecules. The iron(III) oxide in the reactants is surrounded by x water molecules, which leave when the iron(III) oxide is turned into iron. For every one iron(III) oxide that reacts, x water molecules will be formed in the reactants for this reason. However, there are oxygen atoms in the iron(III) oxide which we will balance with water as well. To keep it easier, we will separate these water molecules from the ones surrounding the original iron(III) oxide. Because there are 2 oxygen atoms in the iron(III) oxide and each water molecule has 1 oxygen, we add three waters.
Fe2O3•xH2O(s) ---> 2Fe(l) + 3H2O + xH2O
$$Fe_{2}O_{3}\cdot xH_{2}O(s)\rightarrow2Fe(l)+3H_{2}O+xH_{2}O)$$
Next, we will balance the hydrogen atoms by adding H+ ions to the reactants. Because we added 6 hydrogen atoms when we added 3 water molecules, we must add 6H+ to the reactants.
Fe2O3•xH2O(s) +6H+ ---> 2Fe(l) + 3H2O + xH2O
$$Fe_{2}O_{3}\cdot xH_{2}O(s)+6H^+\rightarrow2Fe(l)+3H_{2}O+xH_{2}O)$$
Finally, we balance the charge by adding electrons. The reactants have a charge of 6+, and the products are neutral, so we add 6 electrons to the reactants to make their overall charge neutral as well.
Fe2O3•xH2O(s) +6H+ + 6e- ---> 2Fe(l) + 3H2O + xH2O
$$Fe_{2}O_{3}\cdot xH_{2}O(s)+6H^{+}+6e^-\rightarrow2Fe(l)+3H_{2}O+xH_{2}O)$$
Now, we balance the oxidation half reaction.
C(s) ---> CO2(g)
$$C(s)\rightarrow CO_{2}(g)$$
The carbon atoms are already balanced, so now we balance the oxygens by adding 2 water molecules to the reactants.
C(s) + 2H2O ---> CO2(g)
$$ C(s)+2H_{2}O\rightarrow CO_{2}(g)$$
We balance the hydrogens by adding 4H+ to the products, as we added 4 hydrogens in the two water molecules.
C(s) + 2H2O ---> CO2(g) + 4H+
$$C(s)+2H_{2}O\rightarrow CO_{2}(g)+4H^+$$
Finally, we balance charge by adding 4 electrons to the products, which have an overall charge of 4+, while the reactants have a neutral charge.
C(s) + 2H2O ---> CO2(g) + 4H+ + 4e-
$$C(s)+2H_{2}O\rightarrow CO_{2}(g)+4H^{+}+4e^-$$
Now, we have our two reactions:
Reduction: Fe2O3•xH2O(s) +6H+ + 6e- ---> 2Fe(l) + 3H2O + xH2O
Oxidation: C(s) + 2H2O ---> CO2(g) + 4H+ + 4e-
$$Reduction: Fe_{2}O_{3}\cdot xH_{2}O(s)+6H^{+}+6e^-\rightarrow2Fe(l)+3H_{2}O+xH_{2}O)$$
$$Oxidation: C(s)+2H_{2}O\rightarrow CO_{2}(g)+4H^{+}+4e^-$$
The last thing we must do is balance the electrons. The reduction reaction gains 6 electrons and the oxidation reaction gives up 4 electrons. The least common multiple of 6 and 4 is 12, so we must multiply the reduction reaction by 2, and the oxidation reaction by 3.
Reduction: 2Fe2O3•xH2O(s) +12H+ + 12e- ---> 4Fe(l) + 6H2O + 2xH2O
Oxidation: 3C(s) + 6H2O ---> 3CO2(g) + 12H+ + 12e-
$$Reduction: 2Fe_{2}O_{3}\cdot xH_{2}O(s)+12H^{+}+12e^-\rightarrow4Fe(l)+6H_{2}O+2xH_{2}O)$$
$$Oxidation: 3C(s)+6H_{2}O\rightarrow 3CO_{2}(g)+12H^{+}+12e^-$$
Q20.9.4
Two solutions, one containing Fe(NO3)2·6H2O and the other containing the same molar concentration of Fe(NO3)3·6H2O, were electrolyzed under identical conditions. Which solution produced the most metal? Justify your answer.
S20.9.4
Electrolysis is the process of applying an external voltage source to drive a nonspontaneous reaction, in this case, the reduction of iron cations into iron metal. Electrons will flow from the anode to the cathode, where iron ions will accept the electrons and be deposited on the electrode.
When Fe(NO3)2·6H2O is electrolyzed, the reduction half reaction that forms iron metal is
Fe2+(aq) + 2e----> Fe(s)
$$Fe^{2+}(aq)+2e^-\rightarrow Fe(s)$$
When Fe(NO3)3·6H2O is electrolyzed, the reduction half reaction that forms iron metal is
Fe3+(aq) + 3e----> Fe(s)
$$Fe^{3+}(aq)+3e^-\rightarrow Fe(s)$$
We can use these half reactions to determine the amount of iron metal produced in the electrolysis process. Because both systems were electrolyzed under identical conditions, we know that the same amount of electrons were transferred.
If x moles of electrons are transferred in the electrolysis of Fe(NO3)2·6H2O, then x/2 moles of Fe metal will be produced.
If x moles of electrons are transferred in the electrolysis of Fe(NO3)3·6H2O, then x/3 moles of Fe metal will be produced.
Therefore, the electrolysis of Fe(NO3)2·6H2O produces the most metal.
Q20.8.3
Why is it important for automobile manufacturers to apply paint to the metal surface of a car? Why is this process particularly important for vehicles in northern climates, where salt is used on icy roads?
S20.8.3
When metal is exposed to oxygen and water, it is oxidized into a metal oxide. Paint helps prevent the metal from coming into contact with oxygen and water, so the oxidation cannot occur.
This is especially important where salt is used on icy roads. If we look at electrochemical cells, a critical part of the system is the salt bridge, which prevents the buildup of charge due to the transfer of electrons. The salt bridge helps to maintain neutrality. Without the salt bridge, the electrolysis will not occur.
Salt on icy roads is an electrolyte that can act as a salt bridge, and help maintain electrical neutrality at the anode and cathode, which increases the rate of corrosion of the car.

