Extra Credit 26
- Page ID
- 82835
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.3.5
Determine the overall reaction and its standard cell potential at 25 °C for the reaction involving the galvanic cell in which cadmium metal is oxidized to 1 M cadmium(II) ion and a half-cell consisting of an aluminum electrode in 1 M aluminum nitrate solution. Is the reaction spontaneous at standard conditions?
S17.3.5
To begin solving this problem, first separate the overall reaction into separate half reactions using the appropriate chemical species mentioned in the problem. You know that Cadmium is in solid metal form, and it is oxidized to its +2 charge ion. You also know that Aluminum Nitrate's aluminum species is reduced and becomes solid Aluminum. Al in Al(NO3)3 is Al3+. Once you figure out which species is oxidized and reduced using their respective oxidation numbers, you can write out the half reactions. Therefore, you obtain the following half reactions:
A - oxidation: Cd(s) --> Cd2+(aq) + 2e-
B - reduction: Al3+(aq) + 3e- --> Al(s)
Next, multiply each half reaction by the correct coefficient to cancel out the electrons. This yields:
A(3) - oxidation: 3Cd(s) --> 3Cd2+(aq) + 6e-
B(2) - reduction: 2Al3+(aq) + 6e- --> 2Al(s)
Add the two balanced half reactions to obtain an overall balanced equation:
3Cd(s) + 2Al3+(aq) --> 3Cd2+(aq) + 2Al(s)
To determine if this reaction is spontaneous at standard conditions, you have to determine the Ecell (Ecathode - Eanode). You know that the cadmium half reaction is the oxidation reaction, so it is the anode. The aluminum reaction is the reduction reaction, so it is the cathode. Using the Standard Reduction Potentials table, you can determine the Ecell of each half reaction.
A - oxidation: Cd(s) --> Cd2+(aq) + 2e- has a standard reduction potential of -0.40V.
B - reduction: Al3+(aq) + 3e- --> Al(s) has a standard reduction potential of -1.66V.
-1.66V - (-0.40V) = -1.26V.
Since Ecell is negative for this cell, the reaction is NON-SPONTANEOUS at standard conditions.
Phase II: This answer is correct. The reaction is nonspontaneous because E° is less than zero, which correlates to a positive ΔG.
Q19.1.24
Predict which will be more stable, [CrO4]2− or [WO4]2−, and explain.
S19.1.24
[CrO4]2- is more stable because Chromium is in the 3d orbital while Tungsten is in the 4d orbital, which has a higher energy level and makes it less stable.
Explanation: According to the rules associated with Crystal Field Stabilizing Energies, stable molecules contain more electrons in the lower-energy molecular orbitals than in the high-energy molecular orbitals. In this case, both complexes have O4 as ligands, and both have a -2 charge. Therefore, you determine stability by comparing the metals. Chromium is in the 3d orbital, according to the periodic table. Tungsten (W) is in the 4d orbital. 3d is a lower energy level than 4d. Therefore, [CrO4]2− is more stable than [WO4]2−.
Phase II: In addition, Tungsten is less stable than Chromium because it is in a lower group. Tungsten is in the 5d orbital and Chromium is in the 3d orbital, meaning that Tungsten has a lower ionization energy. Therefore, the lower the ionization energy, the easier it is to remove an electron, making the compound less stable.
Q12.4.17
Suppose that the half-life of steroids taken by an athlete is 42 days. Assuming that the steroids biodegrade by a first-order process, how long would it take for 164164 of the initial dose to remain in the athlete’s body?
S12.4.17
252 days
\[ \textbf{First Order Reaction: }t_{1/2} = \frac{ln2}{k} = \frac{0.693}{k} \]
\[ \textbf{k} = \frac{ln2}{t_{1/2}} = \frac{ln2}{42 days} = \textbf{1.650}^{\textbf{-2}}s^{-1} \]
\[ A = A_0e^{-kt} \]
\[ \text{[A] = } \frac{1}{64} \text{[A]}_0 \]
\[ \frac{1}{64} \text{[A]}_0 = \text{[A]}_0e^{-0.0165t} \]
\[ \textbf{t = 252 days} \]
Phase II: The answer is correct.
Q21.3.1
Write a brief description or definition of each of the following:
- nucleon
- α particle
- β particle
- positron
- γ ray
- nuclide
- mass number
- atomic number
S21.3.1
(a) A nucleon is a subatomic particle located in the nucleus; essentially, a proton or neutron.
(b) An α particle is one that is emitted during radioactive decay. It is the nucleus of a He atom, and has 4 protons and 2 neutrons. Alpha rays are the least penetrable of all the forms of decay that can be blocked by paper. It is written like this \(_2^4He\)
(c) A β particle is produced from natural radioactive decay. It is a high speed electron, and has an effect of (-1) for the atomic number and 0 for the mass number in a decay equation. It is written like this \(_{-1}^0e\).
(d) A positron is essentially a beta particle with the same 0 mass number, but instead of -1 charge, it gives a +1 charge. It is written like this \(_{+1}^0e\).
(e) Gamma rays are another form of electromagnetic radiation. They have the smallest wavelength of all the particles, but have the highest energy. Gamma rays are the most penetrable of all the forms of decay that can be blocked by lead.
(f) A nuclide is a very specific type of nucleus. It has a certain number of protons and neutrons unique only to itself.
(g) The mass number is the total number of neutrons + protons in an atomic nucleus.
(h) The atomic number is the total number of protons of an element. The total number of protons of an element define the element.
Phase II: The answer is correct. I added some corrections and additional information to each answer.
Q21.7.3
Given specimens uranium-232 (t1/2=68.9y) and uranium-233 (t1/2=159,200y) of equal mass, which one would have greater activity and why?
S21.7.3
Uranium-232 has a greater activity than uranium-233 because activity is inversely proportional to the half life due to the equation \(A=\lambda N\).
Phase II: A general rule for the correlation of activity and half life is that the shorter the half life, the higher the activity.
Q20.4.13
Balance each reaction and calculate the standard electrode potential for each. Be sure to include the physical state of each product and reactant.
1. \(Cl_{2}(g)+H_{2}(g)\rightarrow 2Cl^{-}(aq)+2H^{+}(aq)\)
2. \(Br_{2}(aq)+2Fe^{2+}(aq)\rightarrow 2Br^{-}(aq)+2Fe^{3+}(aq)\)
3. \(2Fe^{3+}(aq)+Cd(s)\rightarrow 2Fe^{2+}(aq)+Cd^{2+}(aq)\)
S20.4.13
Phase II: Problem was mostly complete; I added the SRP chart for reference.
- Write the half Reactions for the equations using the Standard Reduction Potential table:
\[Cl_{2}(g)+2e^{-}\rightarrow 2Cl^{-}(aq); E° = +1.36V\]
\[H_{2}(g)\rightarrow 2H^{+}(aq)+2e^{-}; E° = +0.00V\]
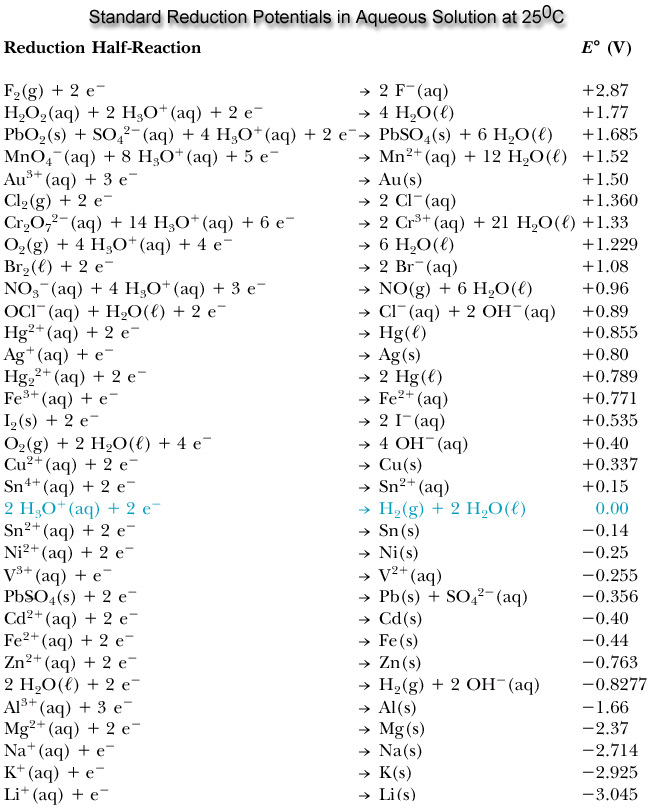
2. Balance the electrons and combine the half reactions in order to find the whole equation. Cancel out any spectator ions (the electrons should cancel out). For this specific reaction, both half reactions have 2 electrons so there is no need to multiply either equation by anything because the electrons will cancel out when the half reactions are added together. All that is left to do is add the half reactions together.
\[Cl_{2}(g)+H_{2}(g)\rightarrow 2Cl^{-}(aq)+2H^{+}(aq)\]
3. In order to find the standard cell potential, use the equation \(E°_{cell}=E°_{cathode}-E°_{anode}\). The cathode is the more positive cell potential of the two, and the anode is the more negative cell potential.
\[=+1.36V - (+0.00V) ⟶ E°_{cell}=+1.36V\]
Use the same steps for the following equations
3. \[(Br_2)_{(aq)} + (Fe^{2+})_{2_(g)} → 2Br^{-}_{(aq)} + Fe^{3+}_{(aq)}\]
Separate into half reactions:
\[Br_{2}(aq)+2e^{-}\rightarrow 2Br^{-}(aq); E° = +1.0873V\]
\[Fe^{2+}(aq)\rightarrow Fe^{3+}(aq)+e^{-}; E° = +0.771V\]
Multiply by coefficient to find the correct number of electrons, then add the equations so the electrons cancel:
\[Br_{2}(aq)+2e^{-}\rightarrow 2Br^{-}(aq)\]
\[2(Fe^{2+}(aq)\rightarrow Fe^{3+}(aq)+e^{-})\]
Total balanced equation: \(Br_{2}(aq)+2Fe^{2+}(aq)\rightarrow 2Br^{-}(aq)+2Fe^{3+}{(aq)}\)
Use the equation and SRP table to find E°:
\[E°_{cell}=E°_{cathode}-E°_{anode}=+1.0873V - (+0.7710V)\]
\[E°_{cell}=+0.3163V\]
3. \(2Fe^{3+}(aq)+Cd(s)\rightarrow 2Fe^{2+}(aq)+Cd^{2+}(aq)\)
Separate into half reactions:
\[Fe^{3+}(aq)+e^{-}\rightarrow Fe^{2+}(aq); E° = +0.771V\]
\[Cd(s)\rightarrow Cd^{2+}(aq)+2e^{-}; E°= -0.403V\]
Multiply by coefficient to find the correct number of electrons, then add the equations so the electrons cancel:
\[2(Fe^{3+}(aq)+e^{-}\rightarrow Fe^{2+}(aq))\]
\[Cd(s)\rightarrow Cd^{2+}(aq)+2e^{-}\]
Total balanced equation: \(2Fe^{3+}(aq)+Cd(s)\rightarrow 2Fe^{2+}(aq)+Cd^{2+}(aq)\)
Use the equation and SRP table to find E°:
\[E°_{cell}=E°_{cathode}-E°_{anode}=+0.771V - (-0.403V)\]
\[E°_{cell}=+1.174V\]
Q20.4.15
Write a balanced chemical equation for each redox reaction.
- H2PO2−(aq) + SbO2−(aq) → HPO32−(aq) + Sb(s) in basic solution
- HNO2(aq) + I−(aq) → NO(g) + I2(s) in acidic solution
- N2O(g) + ClO−(aq) → Cl−(aq) + NO2−(aq) in basic solution
- Br2(l) → Br−(aq) + BrO3−(aq) in basic solution
- Cl(CH2)2OH(aq) + K2Cr2O7(aq) → ClCH2CO2H(aq) + Cr3+(aq) in acidic solution
S20.4.15
Phase II: answers 4 and 5 were not done, so I added those.
General Steps to solving a Redox Reaction:
1. Separate the equation into half reactions
2. Balance the elements other than O and H.
3. Then balance the oxygens by adding water \(H_{2}O\) molecules to the other side of the equation.
4. Balance the hydrogen atoms (be sure to account for any \(H^{+}\) ions that are already in the equation) by adding them to the other side of the equation.
5. Add the appropriate number of electrons in order to balance the charges. Add the \(e^{-}\) to the more positive side of the equation. The number of electrons in both half reactions must be equal so they cancel out, if they are unequal, then multiply by the lowest common multiple in order to balance the equations.
6. Add the half reactions together, cancelling the electrons and extra \(H^{+}\) ions.
Note: If the equation is being balanced in basic solution, then add \(OH^{-}\) everytime you add an extra \(H^{+}\). This will form a water molecule because \(OH^{-} + H^{+} = H_2O\). Then check to see if the equation is balanced.
Answers:
1. \(OH^{-}(aq)+3H_{2}PO_{2}^{-}(aq)+2SbO_{2}^{-}(aq)\rightarrow 3HPO_{3}^{2-}(aq)+2Sb(s)+2H_{2}O(l)\)
2. \(2H^{+}{(aq)}+ 2HNO_{2}{(aq)}+2I^{-}{(aq)}\rightarrow 2NO{(g)}+ I_{2}{(s)}+2H_2O{(l)}\)
3. \(2OH^{-}(aq)+N_{2}O(g)+2ClO^{-}(aq)\rightarrow 2NO_{2}^{-}(aq)+H_{2}O(l)+2Cl^{-}(aq)\)
4. \(6OH^{-}(aq)+3Br_{2}(l)\rightarrow 5Br^{-}(aq)+BrO_3^{-}(aq)+3H_{2}O(l)\)
5. \(3Cl(CH_{2})_{2}OH(aq)+2K_{2}Cr_{2}O_{7}(aq)+16H^{+}(aq)\rightarrow 3ClCH_{2}CO_{2}H(aq)+4Cr^{+3}(aq)+4K^{+}(aq)+11H_{2}O(l)\)
Q20.4.16
Write a balanced chemical equation for each redox reaction.
- I−(aq) + HClO2(aq) → IO3−(aq) + Cl2(g) in acidic solution
- Cr2+(aq) + O2(g) → Cr3+(aq) + H2O(l) in acidic solution
- CrO2−(aq) + ClO−(aq) → CrO42−(aq) + Cl−(aq) in basic solution
- S(s) + HNO2(aq) → H2SO3(aq) + N2O(g) in acidic solution
- F(CH2)2OH(aq) + K2Cr2O7(aq) → FCH2CO2H(aq) + Cr3+(aq) in acidic solution
S20.4.16
Phase II: Problem correct; questions 4 and 5 were not completed.
Look at above general Redox reaction steps.
1. \( I^{-}(aq)+2HClO_{2}(aq)\rightarrow IO_{3}^{-}(aq)+Cl_{2}(g)+H_{2}O(l)\)
2. \(4H^{+}(aq)+O_{2}(g)+4Cr^{2+}(aq)\rightarrow 2H_{2}O(l)+4Cr^{3+}(aq)\)
3. \(2OH^{-}(aq)+2CrO_{2}^{-}(aq)+3ClO^{-}(aq)\rightarrow 2CrO_{4}^{2-}(aq)+H_{2}O(l)+3Cl^{-}(aq)\)
4. \(S(s) + 2HNO_{2}(aq)\rightarrow H_{2}SO_{3}(aq) + N_{2}O(g)\)
5. \(3F(CH_{2})_{2}OH(aq)+2K_{2}Cr_{2}O_{7}(aq)+16H^{+}(aq)\rightarrow 3FCH_{2}CO_{2}H(aq)+4Cr^{+3}(aq)+4K^{+}(aq)+11H_{2}O(l)\)

