Extra Credit 15
- Page ID
- 82822
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q 17.2.4
Balance the following reactions and write the reactions using cell notation. Ignore any inert electrodes, as they are never part of the half-reactions.
- Al(s)+Zr4+(aq)⟶ Al3+(aq)+Zr(s)
- Ag+(aq)+NO(g)⟶ Ag(s)+NO3-(aq)(acidic solution)
- SiO2−3(aq)+Mg(s)⟶ Si(s)+Mg(OH)2(s)(basic solution)
- ClO3−(aq)+MnO2(s)⟶ Cl−(aq)+MnO4−(aq)(basic solution)
Q 17.2.4 Answer
Balancing Redox Reactions
The method to balance redox reactions requires you split the reaction into a reduction half-equation and an oxidation half-reaction. To do this we look at the main element in the compound's oxidation state on reactants side and the products side. If the oxidation state went down (gain of electrons) from reactants side to products side it is an reduction half reaction. If the oxidation state went up (loss of electrons) from reactants side to products side it is the oxidation half reaction. We can remember these two differences by remembering the acronym OIL RIG which stands for Oxidation Is Loss Reduction Is Gain in reference to electrons.
Once you have your two half-reaction you follow these steps for each half-reaction:
- Balance elements other than O and H.
- Balance the oxygen atoms by adding H2O molecules to the opposite side of the equation.
- Balance the hydrogen atoms by adding H+ ions to the opposite side of the equation(do not forget about the hydrogen from the water added in step 2).
- Charges must be the same on both sides therefore add electrons (e-)to the side with a more positive total charge to make charges equal.
- The e- on each of the half reactions must be the same; if they are not the same, you must be multiply the whole half reactions by the lowest common multiple to be make electrons the same.
- Add the half-equations , canceling out the electrons and common terms to form one balanced equation. *
* If the equation is being balanced in a basic solution you add appropriate number of OH- molecules to both sides to make all H+ molecules into water molecules and once again cancelling any common terms.
Writing Reaction in Cell Notation
In order to write the cell notation you will need to know which half-equation is reduction and which is oxidation. Remember that in reduction half-equation there is a gain of electrons and in the oxidation half-reaction there is a lost of electrons.
The cell notation consist of your two half-equations. In it you see a single vertical line that represent a phase change, a comma represents the difference of recatants and products side of one half reaction if no phase change, and a double vertical line that represents a salt bridge. In the left side of salt bridge will be your oxidation half-equation and on the right will be your reduction half-equation. Components come straight from your half-equations in order of left to right. All cell diagrams must start and end with a solid, if half reaction does not have a solid you can use platinum or carbon (graphite) because of its highly unreactive characteristic and can be used as an inert electrode in any reaction. If component is aqueous you must include concentration in parenthesis if it is given, if a gaseous component you must include the pressure in parenthesis if given.
1. Al(s)+Zr4+(aq) ⟶ Al3+(aq)+Zr(s)
Half Reactions
Al(s) ⟶ Al3+(aq) (oxidation)
Zr4+(aq) ⟶ Zr(s) (reduction)
Step 1 Elements other than O and H are already balanced
Step 2 No oxygen atoms so no need to add water molecules
Step 3 No water molecules added so no need to add H+ ions
Step 4 Al(s) ⟶ Al3+(aq)+3e-
Zr4+(aq)+4e- ⟶ Zr(s)
Step 5 4(Al(s) ⟶ Al3+(aq)+3e-)
3(Zr4+(aq)+4e- ⟶ Zr(s))
Step 6 4Al(s)+3Zr4+(aq)+12e- ⟶ 4Al3+(aq)+12e-+3Zr(s)
4Al(s)+3Zr4+(aq) ⟶ 4Al3+(aq)+3Zr(s)
Cell Notation
Al half-equation(oxidation) will be written down first and the the Zr half equation(reduction) will come after the salt bridge. Do not forget the concentrations but since they are not given you do not need to include them.
Al(s)|Al3+||Zr4+|Zr(s)
2.Ag+(aq)+NO(g)⟶ Ag(s)+NO3-(aq)(acidic solution)
Half Reactions
Ag+(aq) ⟶ Ag(s) (reduction)
NO(g) ⟶ NO3-(aq) (oxidation)
Step 1 Elements other than O and H are already balanced
Step 2 No oxygen atoms so no need to add water molecules for first half-equation
NO(g)+2H2O ⟶ NO3-(aq)
Step 3 No water molecules added in the first half-reaction so no need to add H+ ions in first half-equation
NO(g)+2H2O ⟶ NO3-(aq)+4H+
Step 4 Ag+(aq)+e-⟶ Ag(s)
NO(g)+2H2O ⟶ NO3-(aq)+4H++3e-
Step 5 3(Ag+(aq)+e-⟶ Ag(s))
NO(g)+2H2O ⟶ NO3-(aq)+4H++3e-
Step 6 3Ag+(aq)+3e-+NO(g)+2H2O ⟶ 3Ag(s)+NO3-(aq)+4H++3e-
3Ag+(aq)+NO(g)+2H2O⟶ 3Ag(s)+NO3-(aq)+4H+
Cell Notation
NO half-equation(oxidation) will be written down first and the the Ag half equation(reduction) will come after the salt bridge. You Begin with Pt because half-equation does not have a solid.
Pt(s)|NO(g)|NO3-||Ag+|Ag(s)
3.SiO2−3(aq)+Mg(s)⟶ Si(s)+Mg(OH)2(s)(basic solution)
Half Reactions
SiO2−3(aq) ⟶ Si(s) (reduction)
Mg(s) ⟶ Mg(OH)2(s) (oxidation)
Step 1 Elements other than O and H are already balanced
Step 2 SiO2−3(aq) ⟶ Si(s)+2H2O
Mg(s)+2H2O⟶ Mg(OH)2(s)
Step 3 SiO2−3(aq)+4H+ ⟶ Si(s)+2H2O
Mg(s)+2H2O⟶ Mg(OH)2(s)+2H+
Step 4 SiO2−3(aq)+4H++e-⟶ Si(s)+2H2O
Mg(s)+2H2O⟶ Mg(OH)2(s)+2H++2e-
Step 5 2(SiO2−3(aq)+4H++e-⟶ Si(s)+2H2O)
Mg(s)+2H2O⟶ Mg(OH)2(s)+2H++2e-
Step 6 2SiO2−3(aq)+8H++2e-+Mg(s)+2H2O⟶ 2Si(s)+4H2O+Mg(OH)2(s)+2H++2e-
2SiO2−3(aq)+6H++Mg(s)⟶ 2Si(s)+2H2O+Mg(OH)2(s)
*Basic Solution so must add 6OH- to both sides since we have 6H+ left on one side
2SiO2−3(aq)+6H++Mg(s)+6OH-⟶ 2Si(s)+2H2O+Mg(OH)2(s)+6OH-
2SiO2−3(aq)+6H2O+Mg(s)⟶ 2Si(s)+2H2O+Mg(OH)2(s)+6OH-
2SiO2−3(aq)+4H2O+Mg(s)⟶ 2Si(s)+Mg(OH)2(s)+6OH-
Cell Notation
Mg half-equation(oxidation) will be written down first and the the Si half equation(reduction) will come after the salt bridge.
Mg(s),Mg(OH)2(s)|| SiO2−3|Si(s)
4.ClO3−(aq)+MnO2(s)⟶ Cl−(aq)+MnO4−(aq)(basic solution)
Half Reactions
ClO3−(aq)⟶ Cl−(aq) (reduction)
MnO2(s) ⟶ MnO4−(aq) (oxidation)
Step 1 Elements other than O and H are already balanced
Step 2 ClO3−(aq)⟶ Cl−(aq) +3H2O
MnO2(s) +2H2O⟶ MnO4−(aq)
Step 3 ClO3−(aq)+6H+⟶ Cl−(aq) +3H2O
MnO2(s) +2H2O⟶ MnO4−(aq) +4H+
Step 4 ClO3−(aq)+6H++6e-⟶ Cl−(aq) +3H2O
MnO2(s) +2H2O⟶ MnO4−(aq) +4H++3e-
Step 5 ClO3−(aq)+6H++6e-⟶ Cl−(aq) +3H2O
2(MnO2(s) +2H2O⟶ MnO4−(aq) +4H++3e-)
Step 6 ClO3−(aq)+6H++6e-+2MnO2(s) +4H2O⟶ Cl−(aq) +3H2O+2MnO4−(aq) +8H++6e-
ClO3−(aq)+2MnO2(s) +H2O⟶ Cl−(aq) +2MnO4−(aq) +2H+
*Basic Solution so must add 2OH- to both sides since we have 2H+
ClO3−(aq)+2MnO2(s) +H2O+2OH-⟶ Cl−(aq) +2MnO4−(aq) +2H++2OH-
ClO3−(aq)+2MnO2(s) +H2O+2OH-⟶ Cl−(aq) +2MnO4−(aq) +2H2O
ClO3−(aq)+2MnO2(s) +2OH-⟶ Cl−(aq) +2MnO4−(aq) +H2O
Cell Notation
Mn half-equation(oxidation) will be written down first and the the Cl half equation(reduction) will come after the salt bridge. You end with Pt because half-equation does not have a solid.
MnO2(s)|MnO4−||ClO3−(1.0M),Cl−|Pt(s)
Q19.1.13
Find the potentials of the following electrochemical cell:
Cd | Cd2+ (M = 0.10) ‖ Ni2+ (M = 0.50) | Ni
Q19.1.13 Answer
Step 1 Write out your two half reactions and identify which is oxidation and which is reduction using the acronym OIL RIG to remember that oxidation is loss of electrons and reduction is gain of electrons
Cd(s)⟶Cd2+(aq)+2e- (oxidation)
Ni2+(aq)+2e-⟶Ni(s) (reduction)
Step 2 Write out complete balanced equation
Cd(s)+ Ni2+(aq)⟶Cd2+(aq)+Ni(s)
Step 3 Find Eocell
Eocell = Ecathode-Eanode
oxidation: Cd(s)⟶Cd2+(aq)+2e- Eo=-0.40V
reduction: Ni2+(aq)+2e-⟶Ni(s) Eo=-0.26V
* E values come from standard reduction potentials table given above. Also, remember anode is where oxidation happens, and cathode is where reduction happens.
Eocell=-0.26-(-.40)
Eocell=0.14V
Step 4 Find Q
Q=[products]/[reactants] (look at complete balanced equation) (remember that [x] means the concentration of x typically given in molarity and that we ignore solids or liquids)
Q=[Cd2+]/[Ni2+]
Q=0.10/0.50
Q=0.2
Step 5 Calculate E using E= Eocell-(.0592/n)logQ (n is number of moles of electrons transferred and in our case the balanced reaction transfers 2 electrons)
E= 0.14-(.0592/2)log(0.2)
E= 0.14-(-.207)
E=0.1607V
Q19.3.5
Is it possible for a complex of a metal in the transition series to have six unpaired electrons? Explain.
Q19.3.5 Answer
It is not possible to have six unpaired electrons in a complex of a metal in the transition series. This happens because the maximum number of electrons that fit in the d-orbital, which is where unpaired electrons will be for a transition metal, is 10 and to have a full 10, the electrons must be paired two by two for a total of 5 slots for electrons to fill, one spin up and one spin down for each slot. Therefore, according to Hund's Rule which states that all orbitals in a sublevel are singly occupied before any of them are doubly occupied, the maximum number of unpaired electrons in the d-orbital is 5.
Q12.4.5
From the given data, use a graphical method to determine the order and rate constant of the following reaction:
2X⟶Y+Z
| Time (s) | 5.0 | 10.0 | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 | 40.0 |
|---|---|---|---|---|---|---|---|---|
| [X] (M) | 0.0990 | 0.0497 | 0.0332 | 0.0249 | 0.0200 | 0.0166 | 0.0143 | 0.0125 |
Q12.4.5 Answer

| Order | Rate (when we only have one reactant) | Integrated Rate Law |
| 0 | r=k[X]0=k | [X]t=-kt+[X]0 |
| 1 | r=k[X] | ln[X]t=-kt+ln[X]0 |
| 2 | r=k[X]2 | 1/[X]t=kt+1/[X]0 |
Now in order to be able to find the order and rate law of the given data you will have to make three separate graphs:
- [X] vs Time
- ln[X] vs Time
- 1/[X] vs Time
Which ever graph is linear, then that will be the order of reaction and the slope will represent the negative rate constant for zero and first order and it will represent rate constant for second order.
| Time (s) | [X] | ln[X] | 1/[X] |
| 5 | 0.0990 | -2.313 | 10.101 |
| 10 | 0.0497 | -3.002 | 20.121 |
| 15 | 0.0332 | -3.405 | 30.121 |
| 20 | 0.0249 | -3.693 | 40.161 |
| 25 | 0.0200 | -3.912 | 50 |
| 30 | 0.0166 | -4.098 | 60.241 |
| 35 | 0.0143 | -4.247 | 69.930 |
| 40 | 0.0125 | -4.382 | 80 |



As we can see, the graph for second order is the one that is linear. Therefore, the reaction is second order and the rate constant is 1.9965 M-1s-1 (the slope of the line). We get this because the integrated rate law for second order is 1/[X]t=kt+1/[X]0 which can be seen as the equation of a line (y=mx+b) where 1/[X]t is y, 1/[X]0 is b, t is x, and finally k is m which is the slope of the line.
Q12.7.8
- Based on the diagrams in Question Q12.7.6, which of the reactions has the fastest rate? Which has the slowest rate?
- Based on the diagrams in Question Q12.7.7, which of the reactions has the fastest rate? Which has the slowest rate?
Q12.7.8 Answer
Q12.7.6 Diagrams
(a)
(b)
Q12.7.7 Diagrams
(a)
(b)
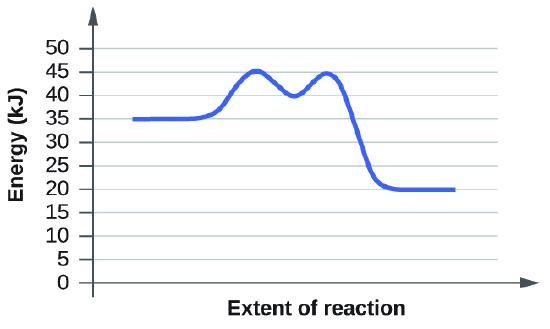
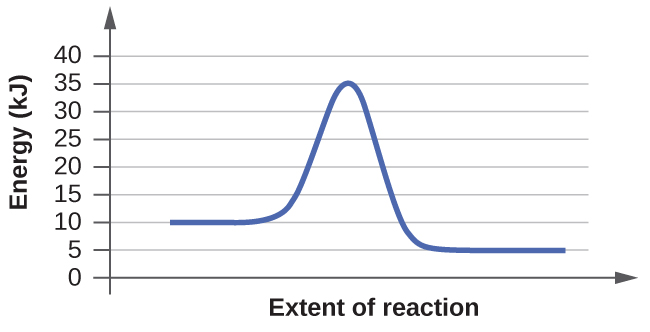
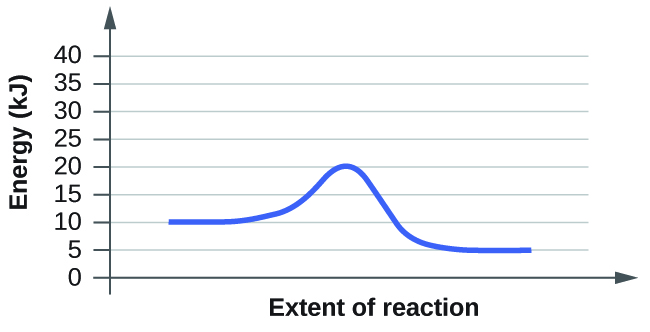
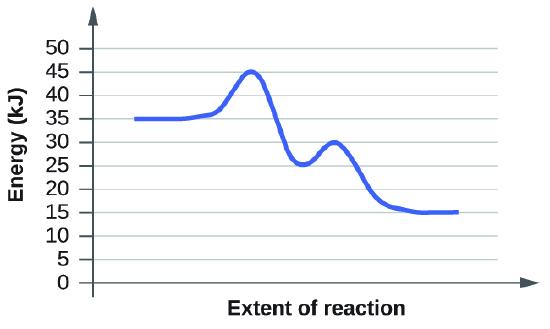
When reading these diagrams, the activation energy can be calculated by taking the vertical distance between the beginning of the blue line to the top of the first hump which represents the rate-limiting transition state. If we know the activation energy we can now predict the fastest rate because activation energy is inversely proportional to rate. If we have a high activation energy we will have a slow rate and if we have low activation energy we will have a fast rate. This happens because low activation energy means we need less energy for molecules to react when they collide.
1. From the first diagram we can see that the vertical distance between beginning and first hump is 25 KJ. On the other hand the second diagram tells us that the activation energy is 10 KJ. Therefore we know that the second diagram has a faster rate since the activation energy is smaller.
2. The first diagram shows us that the vertical distance or activation energy is 10 KJ. The second example tells us that the activation energy is also 10 KJ. Therefore, both of these diagrams have the same rate according to the graphs.
Q21.5.3
Both fusion and fission are nuclear reactions. Why is a very high temperature required for fusion, but not for fission?
Q21.5.3 Answer
Nuclear fission is the splitting of a heavy, unstable nucleus into two lighter nuclei. Nuclear fusion is combining two nuclei to form one. The amount of energy needed for nuclear fission to occur is relatively low because they are unstable nuclei which decay through fission naturally, therefore high temperature is not needed for it to occur. On the other hand, Nuclear fusion needs a huge amount of energy because it requires to bring protons close enough that nuclear forces overcome the very strong electrostatic repulsion. Because such high energy is needed, fusion can only occur in very high temperatures which is the only way there will be enough energy.
Q20.4.1
Is a hydrogen electrode chemically inert? What is the major disadvantage to using a hydrogen electrode?
Q20.4.1 Answer
A hydrogen electrode is chemically inert when properly set up with a specially prepared inert platinum electrode and when it does not participate in the oxidation or reduction half-reactions for that cell. A major disadvantage to using a hydrogen electrode is that it is extremely difficult to keep the pressure of hydrogen gas at exactly 1 bar and in general one needs to make a very advanced apparatus. It is also difficult because hydrogen can be extremely expensive and needs to constantly be used in the electrode for the life of the cell and therefore often called hypothetical because the major disadvantages are too much to get over.
Q20.5.30
Hydrogen gas reduces Ni2+ according to the following reaction: Ni2+(aq) + H2(g) → Ni(s) + 2H+(aq); E°cell = −0.25 V; ΔH = 54 kJ/mol.
- What is K for this redox reaction?
- Is this reaction likely to occur?
- What conditions can be changed to increase the likelihood that the reaction will occur as written?
- Is the reaction more likely to occur at higher or lower pH?
Q20.5.30 Answer
1. Step 1 Split into its half-equations
Ni2+(aq)+2e-→ Ni(s) (reduction)
H2(g)→2H+(aq)+2e- (oxidation)
Step 2 Find Δ Go using Δ Go=-nFEocell
n=2 mol e-
F=96,485 C/mol
Δ Go=-(2 mol e-)(96,485 kJ/V·mol)(-0.25V)
Δ Go=48242.5 J/mol
Step 3 Find K using Δ Go=-RTlnK
R= 8.3145 J /mol·K
T= temperature not given so we use room temp, 298 K
48242.5 J/mol=-(8.3145 J /mol·K)(298K)lnK
48242.5 J/mol=-2477.721 J/mol (lnK)
-19.47=lnK
e-19.47=K
K=3.5 X 10-9
2. Because we found out that Δ Go was positive, we know that the reaction is non-spontaneous. Therefore we can say the reaction is unlikely to occur.
3. Given that ΔH = 54 kJ/mol is positive we know the reaction is endothermic, meaning that heat is on the reactants side. If we were to increase the temperature it would result in the reaction favoring the products since raising temperature would add more reactants and by Le Chatelier's Principle we know that increasing reactants will push a reaction toward the product's side.
4. This reaction will more likely occur in a low PH because that would mean we have a lower H+ concentration. Since H+ is a product in this reaction, lowering its concentration would result in the reaction moving forward because again we look at Le Chatelier's Principle which tells us that when we reduce the amount of products in a reaction, the reaction will move forward to favor those products and in our case make the reaction more likely to happen.


