Extra Credit 12
- Page ID
- 82819
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.1
Write the following balanced reactions using cell notation. Use platinum as an inert electrode, if needed.
- \[Mg(s)+Ni^{_{2+}}(aq)\rightarrow Mg^{_{2+}}(aq)+Ni(s)\]
- \[2Ag^{_{1+}}(aq)+Cu(s)\rightarrow Cu^{_{2+}}(aq)+2Ag(s)\]
- \[Mn(s)+Sn(NO_3)_2(aq)\rightarrow Mn(NO_3)_2(aq)+Au(s)\]
- \[3Cu(NO_3)(aq)+Au(NO_3)_3(aq)\rightarrow 3Cu(NO_3)_2(aq)+Au(s)\]
Q17.2.1
- \[Mg(s)+Ni^{_{2+}}(aq)\rightarrow Mg^{_{2+}}(aq)+Ni(s)\]
- Oxidation is the loss of electrons and reduction is the gain of electrons. Therefore, we can break the given down into the following half reactions.
- Oxidation reaction: \[Mg(s)\rightarrow Mg^{_{2+}}(aq)+2e^{_{-}}\]
- Reduction reaction: \[Ni^{_{2+}}(aq)+2e^{_{-}}\rightarrow Ni(s)\]
- Oxidation takes place in the anode (the left hand side of the cell), and reduction takes place in the cathode (the right hand side of the cell).
- For cell notation, a single line represents a phase change, and the double bars represent the salt bridge.The salt bridge functions to ensure that electrons do not build up and balances to maintain neutrality. The line also indicates a new cell for cell notation.
- \[Mg(s) | Mg^{_{2+}}(aq) || Ni^{_{2+}}(aq) | Ni(s)\]
- \[2Ag^{_{1+}}(aq)+Cu(s)\rightarrow Cu^{_{2+}}(aq)+2Ag(s)\]
- Oxidation is the loss of electrons and reduction is the gain of electrons. Therefore, we can break the given down into the following half reactions.
- Oxidation reaction: \[Cu(s)\rightarrow Cu^{_{2+}}(aq)+2e^{_{-}}\]
- Reduction reaction: \[2Ag^{_{+}}(aq)+2e^{_{-}}\rightarrow 2Ag(s)\]
- \[Ag^{_{+}}(aq)+e^{_{-}}\rightarrow Ag(s)\] This is what the half-reaction would look like prior to multiplying it by two to get the full, balanced equation given to us in the problem.
- Oxidation takes place in the Anode (the left hand side of the cell), and reduction takes place in the Cathode (the right hand side of the cell).
- For cell notation, a single line represents a phase change, and the double bars represent the salt bridge.
- \[Cu(s) | Cu^{_{2+}}(aq) || Ag^{_{+}}(aq) | Ag (s)\]
- \[Mn(s)+Sn(NO_3)_2(aq)\rightarrow Mn(NO_3)_2(aq)+Au(s)\] It seems a tad strange that that the equation was given with Au on the right hand side. This lacks a balance on both sides of the equation and somehow Au is formed from a tin ion. Perhaps one could assume a mistake was made in the question and we can operate with this equation: \[Mn(s)+Sn(NO_3)_2(aq)\rightarrow Mn(NO_3)_2(aq)+Sn(s)\] This creates a balance on both sides and we will be able to use proper half reactions.
- Oxidation is the loss of electrons and reduction is the gain of electrons. Therefore, we can break the given down into the following half reactions.
- Oxidation Reaction: \[Mn(s)\rightarrow Mn^{_{2+}}(aq)+2e^{_{-}}\]
- Manganese has a charge of 2+ on the right side of the oxidation reaction because NO3- has a -1 charge, so Mn must be +2 charge to balance out the ion.
- Reduction Reaction: \[Sn^{_{2+}}(aq)+2e^{_{-}}\rightarrow Sn(s)\]
- Tin has a charge of 2+ on the left side of the reduction reaction because NO3- has a -1 charge, so Sn must be +2 charge to balance out the ion.
- Oxidation takes place in the Anode (the left hand side of the cell), and reduction takes place in the Cathode (the right hand side of the cell).
- For cell notation, a single line represents a phase change, and the double bars represent the salt bridge.
- \[Mn(s) | Mn^{_{2+}}(aq) || Sn^{_{2+}}(aq) | \textbf{Sn}(s)\]
- \[3Cu(NO_3)(aq)+Au(NO_3)_3(aq)\rightarrow 3Cu(NO_3)_2(aq)+Au(s)\]
- Oxidation is the loss of electrons and reduction is the gain of electrons. Therefore, we can break the given down into the following half reactions.
- Oxidation Reaction: \[Cu^{_{3+}}(aq)\rightarrow Cu^{_{2+}}(aq)+1e^{_{-}}\]
- Copper has a +3 and +2 charge because those are the charges needed to balance out the ion since NO3- has a -1 charge
- Reduction Reaction: \[Au^{_{3+}}(aq)+3e^{_{-}}\rightarrow Au(s)\]
- Oxidation takes place in the Anode (the left hand side of the cell), and reduction takes place in the Cathode (the right hand side of the cell).
- For cell notation, a single line represents a phase change, and the double bars represent the salt bridge.
- \[Pt(s) | Cu^{_{+}}(aq), Cu^{_{2+}}(aq) || Au^{_{3+}}(aq) | Au(s)\] (We use Pt as the electrode because it is inert and there is no solid in the oxidation reaction.)
Bold: phase II edits.
Q19.1.10
Would you expect an aqueous manganese(VII) oxide solution to have a pH greater or less than 7.0? Justify your answer.
Q19.1.10
Manganese(VII) oxide, can be written as Mn2O7.
In relation to the Lewis acid-base theory, a Lewis acid accepts lone pair electrons, and is also known as the electron pair acceptor. Based on this theory, acidity can be measured by the element's ability to accept electron pairs. By doing the math, we find that Manganese has an oxidation state of +7 (Oxygen has an oxidation state of -2, and 2x-7=-14 or this can be shown as \(-7(2)+2(x)=0\) and \(x=7\) since the whole compound has a charge of zero, in order to balance the ion's charge, Mn must be +7). Therefore Mn has high capability of accepting electrons due to its high positive charge. For most metals, as the oxidation number increases, so does its acidity, because of its increased ability to accept electrons.
The pH is a logarithmic scale used to measure the acidity and basicity of of solutions based on their H+ ion concentration. Acids have a high H+ concentration, greater than 1.0 X 10-7, while bases have a concentration less than that. Acids have a pH values of less that 7, so Mn2O7 must have a pH less than 7.
Q19.3.2
Draw the crystal field diagrams for [Fe(NO2)6]4− and [FeF6]3−. State whether each complex is high spin or low spin, paramagnetic or diamagnetic, and compare Δoct to P for each complex.
Q19.3.2
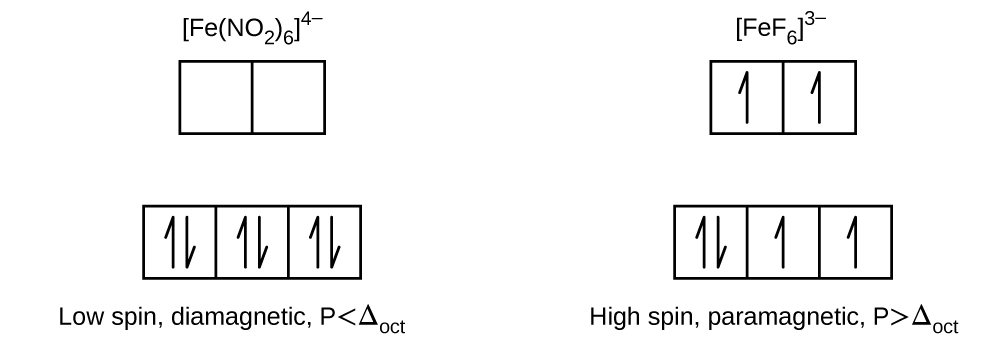
Here are the diagrams with their specific labels of orbitals. The eg's are destabalized in an octahedral field splitting while the t2g's are stabilized. This is based of the concept ligands donating their electrons as Lewis bases and the locations of electrons and nodes per orbital. The corrected version of the latter diagram is given in which should have five electrons and not six since Fe3+ has 5 V.E. :
A) \[[Fe(NO_2)_6]^{_{-4}}\]
- NO2- has a -1 charge. The overall ion has a -4 charge, therefore Fe must be +2 charge. (The math: \(x+(6)(-1)=-4, x+-6=-4, x=+2\) or 2+(6*-1)=-4)
- Fe2+ has 6 valence electrons.
- Next we look at the ligand bonded to Fe, which is NO2- . Based on the spectrochemical Series, NO2- is a strong field ligand meaning that it has a large DELTAo large splitting energy in comparison to the pairing energy, P. So the electrons would rather pair up, as it takes the least amount of energy.
- So [Fe(NO2)6]4− is low spin.
- All 6 electrons are paired up, so it is diamagnetic.
B) \[[FeF_6]^{_{-3}}\]
- F- has a -1 charge. The overall ion has a -3 charge, therefore Fe must be +3 charge. (The math: \(x+(-1)(6)=-3, x+-6=-3, x=+3\) or 3+(6*-1)=-3)
- F3+ has 5 valence electrons.
- Next we look at the ligand bonded to Fe, which is F- . Based on the Spectrochemical Series, F- is a weak field ligand, meaning that it has a small DELTAo or small splitting energy in comparison to the pairing energy, P. So the electrons would rather split up and move up to the higher energy level, rather than pairing up, as it takes the least amount of energy.
- So [Fe(NO2)6]4− is high spin.
- [FeF6]3− is paramagnetic because it has unpaired electrons.
Note: The spectrochemical series shows how ligands affect DELTAo . This is because strong field ligands bind strongly and increase DELTAo and in general binding ability affects DELTAo . Here is the series for reference:

Q12.4.2
Use the data provided to graphically determine the order and rate constant of the following reaction: SO2Cl2 ⟶ SO2+Cl2
| TIME (s) | 0 | 5.00 × 103 | 1.00 × 104 | 1.50 × 104 | 2.50 × 104 | 3.00 × 104 | 4.00 × 104 |
| [SO2Cl2] (M) | 0.100 | 0.0896 | 0.0802 | 0.0719 | 0.0577 | 0.0517 | 0.0415 |
Q12.4.2
This is what the [SO2Cl2] vs. Time (s), ln[SO2Cl2] vs. Time (s), and 1/[SO2Cl2] graphs should look like once all the points are plotted. Based on the analysis of the graphs and their slopes, we know that the data describes a zero order reaction. To clarify, for a zero order reaction the plot for concentration vs. time should be linear. Then, for a first order the ln of concentration should be and, for second order reaction, the plot of (1/ concentration) should be.
A zero order reaction has a negative slope on [A] vs. time graphs, and is the plot that produces a straight line.
Here are graphs for each of the reaction orders with regression lines and their r squared values. The r2 value quantifies how linear (correlated) the data is, where closest to one means most linear. So, since the first order reaction has an r2 value of 1 and appears the most linear, the reaction is first order.
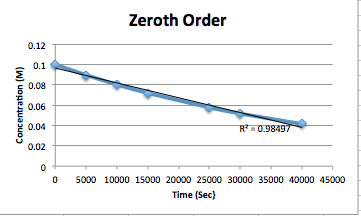


Since it is a first order reaction, we can assume the rate law is: \(k[SO_{2}Cl_{2}]\). K here is the rate constant. We can then find k. Based upon the equation for first order reactions: \([A]=[A_0]e^{-kt}\) and the graph depicted of ln[SO2Cl2] vs time, to see the slope is -k. So we can find the slope by taking the change in y over change in x of any two points on the line to get -k. Then, we make the value positive to get the actual k: \(slope=\frac{\Delta ln[NO_2 Cl_2]}{\Delta t}\) \(slope=\frac{ln[NO_2Cl_2]_{5000}-ln[NO_2Cl_2]_0}{5000-0}\) \(slope=\frac{ln[.0869M]-ln[.100M]}{5000s-0s}=-2.20x10^{-5}1/s\). This is the value for -k. So multiplying by -1 gives the rate constant, k= 2.20x10-5 1/s. We know units are 1/sec based on the reaction order. This is derived by: \(\frac{M^1}{M^{1}sec}=\frac{1}{sec}\). Note that M= mol/L.
To solve for k, we can use the formula Rate=k[SO2Cl2] because it is a zero order reaction. Rearrange the equation to k=rate/[SO2Cl2]. Then convert the time values into rate values, Rate=1/time.
- So for time 0, Rate=1/0=0,
- for time 5.00 × 103, Rate=1/(5.00 × 103)=2E-4
- for time 1.00 × 104, Rate=1/(1.00 × 104)=1E-4
- for time 1.50 × 104, Rate=1/(1.50 × 104)=6.667E-5
- for time 2.50 × 104, Rate=1/(2.50 × 104)=4E-5
- for time 3.00 × 104, Rate=1/(3.00 × 104)=3333.33
- for time 4.00 × 104, Rate=1/(4.00 × 104)=2.5E-5
We use 2 points of data to find the value of k.
k=(Rate1-Rate2)/[SO2Cl2]1-[SO2Cl2]2
k=(2E-4 - 2.5E-5)/(0.0896 - 0.0415)= 1.75E-4/0.481= 3.4x10-3 mol/L*S
The units of K is determined by the reaction order, and can be found using the formula below. \[k=(conc. units)^{_{w-1}}(time)^{_{-1}}\]
The bold and the graphs by it are done by the phase II.
Q12.7.5
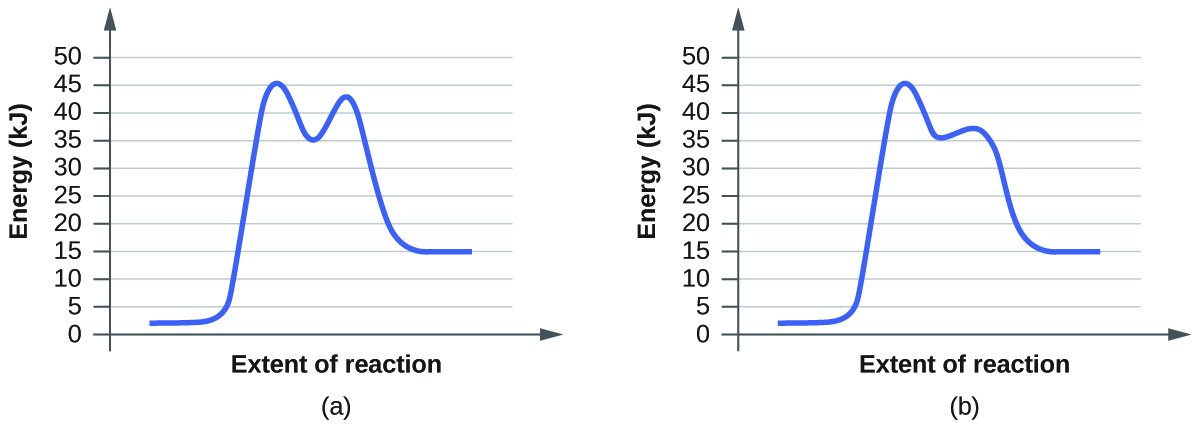
For each of the following pairs of reaction diagrams, identify which of the pairs is catalyzed:
(a)
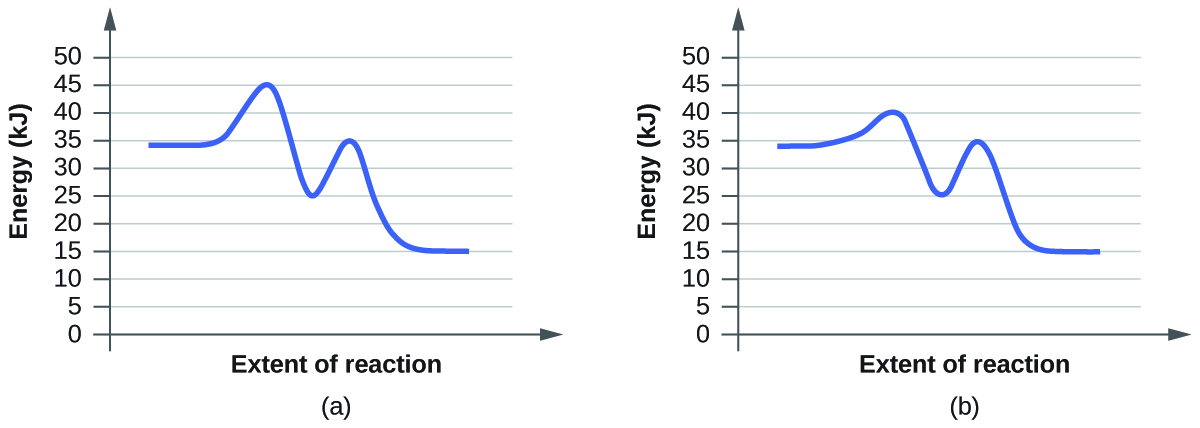
(b)
Q12.7.5
You can identify the effect of a catalyst in a reaction based on the lowering of the transition state's energy. Transition state is the state with the highest energy in the reaction. (a) A; (b) B. The reason the transition state is identifiable by the the highest energy is because that is the state with the highest activation energy. The collision theory states that particles must collide with sufficient energy in order to react. This energy is called the activation energy. The function of a catalyst is to lower this activation energy. The highest transition state is rate limiting transition state since it is the highest barrier.
Q21.4.28
Write a balanced equation for each of the following nuclear reactions:
- Mercury-180 decays into Platinum-176
- Zirconium-90 and an electron are produced by the decay of an unstable nucleus
- Thorium-232 decays and produces an alpha particle and a Radium-228 nucleus, which decays into Actinium-228 by beta decay
- Neon-19 decays into Fluorine-19
Q21.4.28
In nuclear chemistry, it is important to write equations where mass number and atomic number are balanced on both sides of the equation: \[{^A_Z}Element Symbol\]
In general, Z denotes atomic number (proton count)vand A denotes the number neutrons and protons in total.
- Mercury-180 decays into Platinum-176
-
Based on Periodic table, Mercury-180 has 80 protons, and 180 nucleons. In order to decay into Platinum-176, it must lose 4 nucleons and 2 protons, because Platinum has 78 protons.
-
Therefore, it must be alpha decay
-
\[{^{180}_{80}Hg}\rightarrow {^{4}_2He}+{^{176}_{78}Pt}\]
-
- Zirconium-90 and an electron are produced by the decay of an unstable nucleus
-
Based on the Periodic Table, Zirconium-90 has 90 nucleons and 40 protons. If decay produces an electron (which has no mass and a charge of -1), both Zr and the electron must be on the same side.
-
Therefore, it must be Beta particle production.
-
\[{^{90}_{39}Y}\rightarrow {^{90}_{40}Zr}+{^{0}_{-1}e^{_-}}\]
-
- Thorium-232 decays and produces an alpha particle and a Radium-228 nucleus, which decays into Actinium-228 by beta decay
-
\[{^{232}_{90}Th}\rightarrow {^{4}_{2}He^{_-}}+ {^{0}_{-1}e^{_-}}+{^{228}_{89}Ac}\]
-
This can be written as two separate equations so A and Z balance on both sides.
-
\[{^{232}_{90}Th}\rightarrow {^{4}_{2}He}+{^{228}_{88}Rd}\] \[ {^{228}_{88}Rd}\rightarrow {^{0}_{-1}e^{_-}}+{^{228}_{89}Ac}\]
-
- Neon-19 decays into Fluorine-19
-
Based on the periodic table, Neon-19 has 19 nucleons, and 10 protons.
-
In order to decay into Fluorine-19, which has 19 nucleons and 9 protons, Neon must lose one proton. Therefore it must be Beta decay, electron capture.
-
\[{^{19}_{10}Ne}+{^{0}_{-1}e^{_-}}\rightarrow {^{19}_{9}F}\]
-
Q20.3.14
For each redox reaction, write the half-reactions and draw the cell diagram for a galvanic cell in which the overall reaction occurs spontaneously. Identify each electrode as either positive or negative.
- \[Ag(s)+Fe^{_{3+}}(aq)\rightarrow Ag^{_{+}}(aq)+Fe^{_{2+}}\]
- \[Fe^{_{3+}}+1/2H_2(g)\rightarrow Fe^{_{2+}}(aq)+H^{_{+}}(s)\]
Q20.3.14
1. \[Ag(s)+Fe^{_{3+}}(aq)\rightarrow Ag^{_{+}}(aq)+Fe^{_{2+}}\]
- Oxidation is the loss of electrons and reduction is the gain of electrons. Therefore, we can break the given down into the following half reactions.
- Oxidation half reaction: \[Ag(s)\rightarrow Ag^{_{+}}(aq)+e^{_{-}}\]
- Reduction half reaction: \[Fe^{_{3+}}(aq)+e^{_{-}}\rightarrow Fe^{_{2+}}(s)\]
- Oxidation takes place in the anode (LHS of the cell) and reduction takes place in the cathode (RHS of the cell).
- Cell diagram: \[Ag(s) | Ag^{_{+}}(aq) || Fe^{_{3+}}(aq), Fe^{_{2+}}(aq) | Pt(s)\]
- Use Platinum as the electrode because it is inert, and their are no solids present in the reduction reaction.
- The anode (left hand side) is the negative electrode because the electrons are flowing from the anode to the cathode.
2. \[Fe^{_{3+}}+1/2H_2(g)\rightarrow Fe^{_{2+}}(aq)+H^{_{+}}(s)\]
- It may be helpful to rewrite the reaction as: \[2Fe^{_{3+}}+1H_2(g)\rightarrow 2Fe^{_{2+}}(aq)+2H^{_{+}}(s)\]
- Oxidation is the loss of electrons and reduction is the gain of electrons. Therefore, we can break the given down into the following half reactions.
- Oxidation half reaction: \[H_2(g)\rightarrow 2H^{_{+}}(aq)+2e^{_{-}}\]
- Reduction half reaction: \[2Fe^{_{3+}}(aq)+2e^{_{-}}\rightarrow 2Fe^{_{2+}}(s)\]
- Oxidation takes place in the anode (LHS of the cell) and reduction takes place in the cathode (RHS of the cell).
- Cell Diagram: \[Pt(s)[H_2(g)] | 2H^{_{+}}(aq) || Fe^{_{2+}}(aq), Fe^{_{3+}}(aq) | Pt(s)\]
- Use Platinum as the electrode in the cathode because it is inert, and their are no solids present in the reduction reaction.
- Use SHE (Standard Hydrogen Electrode) as the electrode in the anode.
- The anode (left hand side) is the negative electrode because the electrons are flowing from the anode to the cathode.
For these reactions the anode would be negative and the cathode would be positive. This is because at the anode electrons are released there is a buildup of electrons until the electrochemical gradient is reached.
Q20.5.27
Under acidic conditions, ideally any half-reaction with E° > 1.23 V will oxidize water via the reaction O2(g) + 4H+(aq) + 4e− → 2H2O(l).
- Will aqueous acidic KMnO4 evolve oxygen with the formation of MnO2?
- At pH 14.00, what is E° for the oxidation of water by aqueous KMnO4 (1 M) with the formation of MnO2?
- At pH 14.00, will water be oxidized if you are trying to form MnO2 from MnO42− via the reaction 2MnO42−(aq) + 2H2O(l) → 2MnO2(s) + O2(g) + 4OH−(aq)?
Q20.5.27
- Write the reduction potential reaction for KMnO4 with the formation of MnO2.
- It looks like: \[{MnO_{_4}}^{_{-}}+4H^{_{+}}(aq)+3e^{_{-}}\rightarrow MnO_{_{2}}(s)+2H_2O\]
- Based on the standard reduction potentials, E°=1.68V.
- E°cell= E°cathode - E°anode
- The MnO4 gets reduced, therefore it is the cathode, and water is oxidized, so it is the anode.
- E° = 1.68-1.23= 0.45 V, so yes.
- We use the Nernst equation since it involves the relationship between pH and voltage: \[E = E^{_{º}}- \frac{0.0592}{n}logQ\]
- \[E^{_{º}}=1.23V\]
- n=3 because there are 3 moles of electrons transferred from the reaction: \[{MnO_{_4}}^{_{-}}+4H^{_{+}}(aq)+3e^{_{-}}\rightarrow MnO_{_{2}}(s)+2{H_2O}\]
- Q is the reaction quotient. Liquids, and solids are not considered in the calculation, so you only consider the concentration of Hydrogen.
- \[Q=\frac{[products]}{[reactants]}\] , where "x" and "y" are the coefficients of the products and reactants.
- pH=-log[H+], and the pH given is 14.
- Now we plug everything into the Nernst equation: \[E=1.23-\frac{0.0592}{3}log{10^{_{14}}}^{4}\]
- The equation should actually be \[E=.45-\frac{0.0592}{3}log{(1x10^{14})}\]. So E=.1737
- We use .45V because it is the standard reduction potential we found in the first part. Though the problem says to find the standard reduction potential, being given pH lends itself to the Nernst equation.
- We get E=0.1231V
- To determine whether water will be oxidized or not, we need to compare the values of the reduction potentials of \[{O_{_2}}(g) + 4H^{_{+}}(aq) + 4e^{_{-}} \rightarrow 2H_2O(l)\] and \[{MnO_{_4}}^{_{-}}+4H^{_{+}}(aq)+3e^{_{-}}\rightarrow MnO_{_{2}}(s)+2H_2O\]
We can conclude that water is oxidized because the reduction potential of water (1.23v) is lower that the reduction potential of MnO4- (1.68v).
We would use the equations: \[MnO_4^{2-}(aq)+2H_{2}O+2e^{-}\rightarrow MnO_{2}(aq)+4OH^{-}(aq)\] and \[4H^{+}(aq)+O_{2}(g)+4e^{-}\rightarrow 2H_{2}O(l)\].
And then we can multiply top equation by 2 and add 4OH- on each side of the bottom equation to get the overall equation. Based on the equations we see water is oxidized. After writing half reaction reduction equations we see the SRP's are .60v and 1.23v. Then if we do cathode minus anode we get -.63V which shows a lack of spontaneity and so water will not be oxidized. SRP from: (mmsphyschem.com).

