Extra Credit 11
- Page ID
- 82818
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.1.10
Why must the charge balance in oxidation-reduction reactions?
A:
In redox reactions, the oxidation half-reactions lose electrons, while the reduction half-reactions gain electrons. The electrons lost by the oxidation half-reactions should all be gained by the reduction half-reactions. The number of electrons and the electric charge in the overall reactions does not change. Therefore, the charge in redox reactions must be balanced.
Q19.1.9
Why is the formation of slag useful during the smelting of iron?
A:
Slag is a co-product of the reduction process of iron. The slag layer prevents the iron from contacting the oxygen and re-oxidizing.
Q19.3.1
Determine the number of unpaired electrons expected for [Fe(NO2)6]3−and for [FeF6]3− in terms of crystal field theory.
A:
NO2- is a strong field ligand and generates a high Δo, while F- is a weak field ligand and therefore generates a low Δo. Therefore, [Fe(NO2)6]3− will have a low spin configuration and [FeF6]3− will have a high spin configuration. Fe3+ has 5 d electrons. [Fe(NO2)6]3− as a low spin configuration will have all 5 d electrons in the 3 t2g orbitals, which leaves only 1 unpaired electron. [FeF6]3− as a high spin configuration will have the 5 d electrons in all d orbitals, including both the t2g orbitals and the eg orbitals, with 1 electron in each orbital. It will have 5 unpaired electrons in [FeF6]3− .
Q12.4.1
Describe how graphical methods can be used to determine the order of a reaction and its rate constant from a series of data that includes the concentration of A at varying times.
A:
Three graphs can be made from the data that includes the concentration of A at varying times to determine the order of the reactions: [A] vs. time, ln[A] vs. time, and 1/[A] vs. time.
For zero-order reactions, [A] = kt, and the graph of [A] vs. time should appear to be a straight line, where k equals to the slope of the line.
For first-order reactions, ln([A]/[A]0) = - kt, and the graph of ln[A] vs. time should appear to be a straight line, where - k equals to the slope of the line.
For second-order reactions, 1/[A] = kt + 1/[A]0, and the graph of 1/[A] vs. time should appear to be a straight line, where k equals to the slope of the line.
Q12.7.4
For each of the following pairs of reaction diagrams, identify which of the pair is catalyzed:
(a)
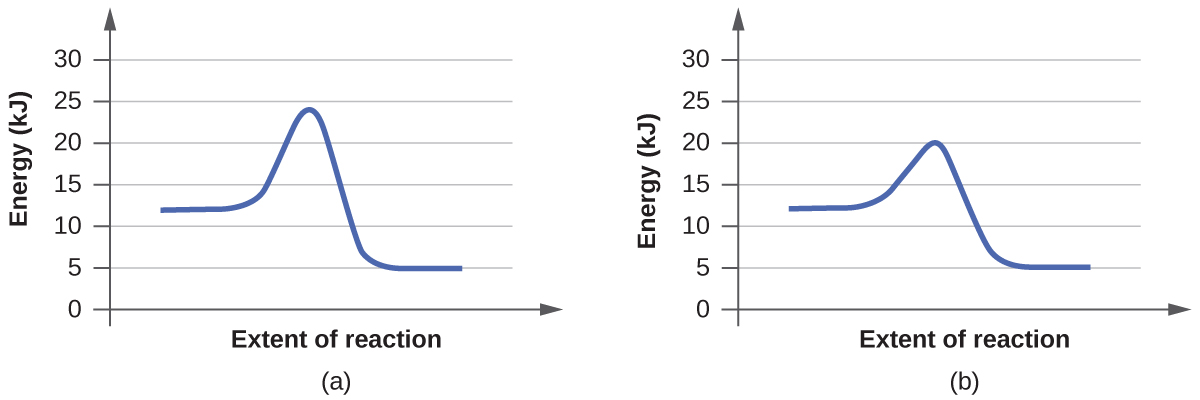
(b)
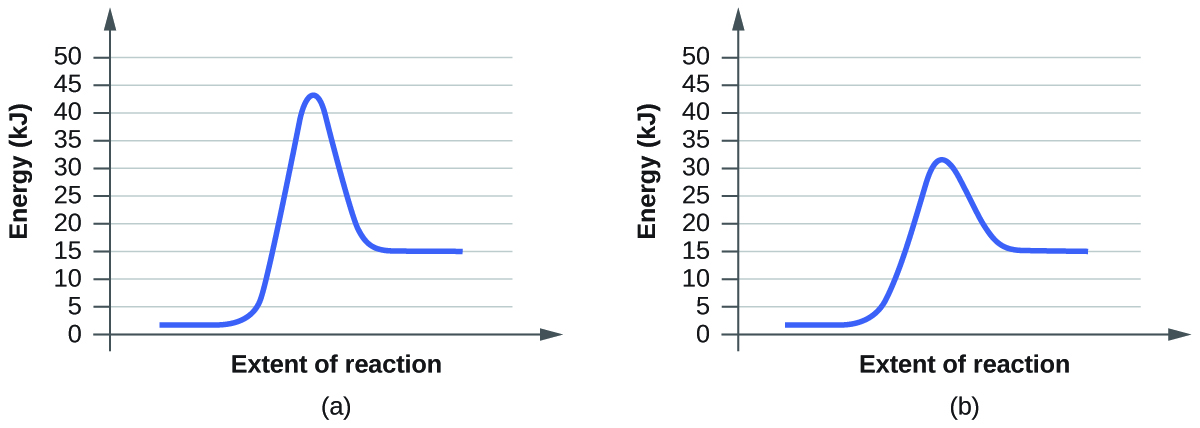
A:
For Pair (a), graph (b) has lower activation energy. Therefore reaction (b) is the catalyzed reaction.
For Pair (b), graph (b) also has lower activation energy. Therefore reaction (b) is the catalyzed reaction.
Q21.4.27
Write a balanced equation for each of the following nuclear reactions:
- bismuth-212 decays into polonium-212
- beryllium-8 and a positron are produced by the decay of an unstable nucleus
- neptunium-239 forms from the reaction of uranium-238 with a neutron and then spontaneously converts into plutonium-239
- strontium-90 decays into yttrium-90
A:
1. 21283Bi → 21284Po + 0-1β
2. 85B → 84Be + 01β
3. 23892U + 10n → 23993Np + 0-1β → 23994Pu + 20-1β
4. 9038Sr → 9039Y + 0-1β
Q20.3.13
For each galvanic cell represented by these cell diagrams, determine the spontaneous half-reactions and the overall reaction. Indicate which reaction occurs at the anode and which occurs at the cathode.
- Zn(s)∣Zn2+(aq) ∥ H+(aq)∣H2(g), Pt(s)
- Ag(s)∣AgCl(s)∣Cl−(aq) ∥ H+(aq)∣H2(g)∣Pt(s)
- Pt(s)∣H2(g)∣H+(aq) ∥ Fe2+(aq), Fe3+(aq)∣Pt(s)
A:
- reduction: 2H+(aq) + 2e− → H2(aq); cathode
oxidation: Zn(s) → Zn2+(aq) + 2e−; anode
overall: Zn(s) + 2H+(aq) → Zn2+(aq) + H2(aq)
- reduction: 2H+(aq) + 2e− → H2(aq); cathode
oxidation: Ag(s) + Cl−(aq) → AgCl(s) + e−; anode
overall: 2Ag(s) + 2H+(aq) + 2Cl−(aq) → H2(g)+ 2AgCl(s)
- reduction: Fe3+(aq) + e− → Fe2+(aq); cathode
oxidation: H2(g) → 2H+(aq) + 2e−; anode
overall: 2Fe3+(aq) + H2(g) → 2H+(aq) + 2Fe2+(aq)
Q20.5.26
Ideally, any half-reaction with E° > 1.23 V will oxidize water as a result of the half-reaction O2(g) + 4H+(aq) + 4e− → 2H2O(l).
- Will FeO42− oxidize water if the half-reaction for the reduction of Fe(VI) → Fe(III) is FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O; E° = 1.9 V?
- What is the highest pH at which this reaction will proceed spontaneously if [Fe3+] = [FeO42−] = 1.0 M and PO2PO2= 1.0 atm?
A:
1. E° = 1.9 V > 1.23 V
Therefore, FeO42− will oxidize water.
2. Anode - oxidation: 2H2O(l) → O2(g) + 4H+(aq) + 4e−; E° = 1.23 V
Cathode - reduction: FeO42−(aq) + 8H+(aq) + 3e− → Fe3+(aq) + 4H2O; E° = 1.9 V
Overall reaction: 4FeO42−(aq) + 20H+(aq) → 3O2(g) + 4Fe3+(aq) + 10H2O; E = Ecathode - Eanode = 0.67 V
E = E° - 0.0592 V/n logQ
E > 0 V; n = 12; E° = 0.67 V
Q = 1/[H+]20 < 10135.81
[H+]minimum =
*** Apparently this number is too large and my calculator says "overflow" when I put it in. I may have done this problem wrong, but if I did not, I am pretty sure that this is step is as close as I can get to the solution... :(

