Extra Credit 25
- Page ID
- 96909
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q16.4.21
Consider the decomposition of CaCO3(s) into CaO(s) and CO2(g). What is the equilibrium partial pressure of CO2 at room temperature?
The equation for this reaction is
\(\ce{CaCO3_{(s)}} \leftrightharpoons \ce{CaO_{(s)}} + \ce{CO2_{(g)}}\)
And the equilibrium constant is
$$ K_p = {\ce{[CO2]}} $$
To calculate the partial pressure of CO2, Kp must first be calculated with the equation \(\Delta G^\circ_{rxn} = -RT\ln(K_p)\). \(\Delta G = 0\) for this reaction since it is at equilibrium. The \(\Delta G^\circ_{rxn}\) and be calculated with the \(\Delta G^\circ_{f}\) for each species
\(\ce{CaCO3_{(s)}}: \Delta G^\circ_{f} = -1128.8 (kJ/mol)\)
\(\ce{CaO_{(s)}}: \Delta G^\circ_{f}\) = -604.2 (kJ/mol)\)
\(\ce{CO2_{(g)}}: \Delta G^\circ_{f}\) = -394.6 (kJ/mol)\)
$$ \Delta G^\circ_{rxn} = {((-604.2) + (-394.6)) - (-1128.8)} $$
$$ \Delta G^\circ_{rxn} = {130 kJ/mol} $$
We know \(T = 25^\circ C (298 K)\) and R = 8.314 J/mol*K as imputing these values into the equation above will determine the equilibrium constant
$$ \Delta G^\circ_{rxn} = {-RT\ln(K_p)} $$
$$ 1.3*10^{5} = {-(8.314)(298)\ln{K_p}} $$
$$ K_p = {1.63*10^{-23} = \ce{[CO2]}} $$
It makes sense that the partial pressure of the CO2 would be so small since CaCO3 does not readily dissociate so there would be a very small amount of CO2 produced from this reaction.
Q15.1.X
Which of the following compounds precipitates from a solution that has the concentrations indicated? (See Table E3 for Ksp values.)
- KClO4: [K+] = 0.01 M,
\(\ce{[ClO4- ]}\) = 0.01 M - K2PtCl6: [K+] = 0.01 M,
\(\ce{[PtCl6^2- ]}\) = 0.01 M - PbI2: [Pb2+] = 0.003 M, [I–] = 1.3
× 10–3 M - Ag2S: [Ag+] = 1
× 10–10 M, [S2–] = 1× 10–13 M
Solution:
For the compound to precipitate from the concentrations given, Q>K so that the reaction will shift to the left to form the precipitate. For each compound, Q much be calculated and compared to the Ksp value given in Table E3. Since each compound is a solid, The Equilibrium constant will be a product of the reactants.
a. \(\ce{KClO^4_{(s)}} \leftrightharpoons {K^+_{(aq)}} + {ClO^{4-}_{(aq)}}\)
\(Q= \ce{[ClO4^{-}]}\)\(\ce{[K+]}\) \(Q = (0.01)(.01) = 1.0*10^{-4}\)
\(K_{sp} = 1.05*10^{-2}\) \(Q<K\)
This solution would not produce the precipitate
b. \(\ce{K_2PtCl_{6(s)}} \leftrightharpoons {2K^+_{(aq)}} + {PtCl^{2-}_{6(aq)}}\)
\(Q= \ce{[2K+]^2}\)\(\ce{[PtCl6^2- ]}\) \(Q = (.01)^2(.01) = 1.0*10^{-6}\)
\(K_{sp} = 7.48*10^{-6}\) \(Q<K\)
This solution will not percipitate to form the compound
c. \(\ce{PbI_{2(s)}} \leftrightharpoons {Pb^{2+}_{(aq)}} + {2I^-_{(aq)}}\)
\(Q= \ce{[Pb^{2+}]}\)\(\ce{[I^-]^2}\) \(Q = (.003)(1.3*10^{-3})^2 = 5.1*10^{-9}\)
\(K_{sp} = 9.8*10^{-9}\) \(Q<K\)
This solution will not percipitate
d. \(\ce{Ag_2S_{(s)}} \leftrightharpoons {2Ag^+_{(aq)}} + {S^{2-}_{(aq)}}\)
\(Q= \ce{[Ag+]^2}\)\(\ce{[S^2- ]}\) \(Q = (1.0*10^{-10})^2(1.0*10^{-13}) = 1.0*10^{-33}\)
\(K_{sp} = 6.3*10^{-50}\) \(Q>K\)
Since K>Q, the reaction will shift to the left and precipitate to form the compound Ag2S.
Q14.6.5
What is [H3O+] in a solution of 0.075 M HNO2 and 0.030 M NaNO2?
\(\ce{HNO2_{(aq)}}+\ce{H2O_{(l)}}⇌\ce{H3O+_{(aq)}}+\ce{NO2^-_{(aq)}} \hspace{20px} K_\ce{a}=4.5×10^{−5}\)Solution:
To find the concentration of hydronium ions for this buffer solution, an ICE table will be used to calculate the concentrations at equilibrium with the equilibrium constant given. HNO2 is a weak acid so it will only partially dissociate, and NaNO2 will completely dissociate into its ions.
| \(\ce{[HNO2]}\) | \(\ce{[H3O+]}\) | \(\ce{[NO2^-]}\) | |
| Initial | \(0.75 M\) | \(0\) | \(0.030M\) |
| Change | \(-x\) | \(+x\) | \(+x\) |
| Equilibrium | \(0.75-x\) | \(x\) | \(0.03+x\) |
The equilibrium constant equation is
$$K_a = {\ce{[H3O+]}\ce{[NO2^{-}]} \over \ce{[HNO2]}} $$
And with the Ka provided and the equilibrium values from the ICE table, we get the equation
$$4.5*10^{-5} = {(x)(.03+x) \over (0.75 -x)} $$
When solved using a graphing calculator or the quadratic formula, the resulting solution is
\(x=0.0011\)
which when plugged back into the equilibrium value for the concentration of hydronium ions equals
\(\ce{[H3O+]} = 0.0011\)
Q13.3.13
Pure iron metal can be produced by the reduction of iron(III) oxide with hydrogen gas.
- Write the expression for the equilibrium constant (Kc) for the reversible reaction \[\ce{Fe2O3}(s)+\ce{3H2}(g)\rightleftharpoons\ce{2Fe}(s)+\ce{3H2O}(g) \hspace{20px} ΔH=\mathrm{98.7\:kJ}\]
- What will happen to the concentration of each reactant and product at equilibrium if more Fe is added?
- What will happen to the concentration of each reactant and product at equilibrium if H2O is removed?
- What will happen to the concentration of each reactant and product at equilibrium if H2 is added?
- What will happen to the concentration of each reactant and product at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?
- What will happen to the concentration of each reactant and product at equilibrium if the temperature of the system is increased?
Solution:
a. \(\ce{Fe_{(s)}}\) and \(\ce{Fe2O3_{(s)}}\) will be left out of the equilibrium expression since they are solids. The equilibrium expression is as follows:
$$K_c = { \ce{[H2O]^3} \over \ce{[H2]^3} } $$
b. Since Fe is a solid adding more of it will not affect the equilibrium so there will be not change in the concentrations of the reactants or the products.
c. If H2O is removed, Q will become less than K so reactants will decrease and products will increase to reestablish equilibrium.
d. If H2 is added, Q will become less then K so the reactant concentrations will decrease and product concentrations will increase to reestablish equilibrium concentration.
e. If the pressure is increased, the reactants will be favored since there is 4 moles of reactants and 5 moles of products, so reactant concentrations will increase and product concentrations will decrease.
f. Since \(ΔH=\mathrm{98.7\:kJ}\), the reaction is endothermic, so an increase in temperature would increase K and cause an increase in concentrations of reactants and a decrease in concentration of products.
Q16.4.25B
Acetic acid, CH3CO2H, can form a dimer, (CH3CO2H)2, in the gas phase.
\[\ce{2CH3CO2H}(g)⟶\ce{(CH3CO2H)2}(g)\]
The dimer is held together by two hydrogen bonds with a total strength of 66.5 kJ per mole of dimer.
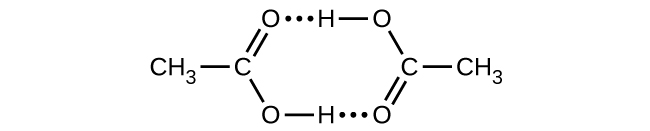
At 25 °C, the equilibrium constant for the dimerization is 1.3
To caculate the \(\Delta S^\circ\) for the reaction, the equation
$$ \Delta G^\circ = {-RT\ln{K_p}} $$
can be substituted into the equation
$$ \Delta G^\circ = {\Delta H^\circ - T\Delta S^circ } $$
to get the equation which can be used to solve for \(\Delta S^\circ\) for the reaction with the information given
$$ \Delta H^\circ - T\Delta S^\circ = {-RT\ln{K_p}} $$
$$ \Delta S ^\circ = {-RT\ln{K_p} - \Delta H ^\circ \over T} $$
\(K_p = 1.3*10^3\) is given and \(\Delta H^\circ = -66.5 kJ/mol\) since that is the change in enthalpy when 1 mole of the dimer is formed from each molecule of acetic acid. Inputting these into the equation equals:
$$ \Delta S^\circ = {-(8.314)(298)\ln{(1.3*10^3)} - (-6.65*10^4) \over (298)} $$
$$ \Delta S^\circ = {163.5 J/mol} $$
This reaction is both entropically and enthaplically favorable so it is a spontaneous reaction.

