6.13: Transition Elements
- Page ID
- 53705
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)

What are the similarities and differences between these two cars?
From the outside, the two cars below look the same (except for the flashy paint job on the racing model). They are the same model of the car, but one is a stock edition for regular driving, while the other one is built for high-speed racing. We really can't tell much from the external view. To see the differences, we need to go under the hood, take the engines apart, and look at the braking and suspension systems in order to see how the two cars differ.
Many electron configurations of elements are simple and straightforward. We can look at the outer shell and easily understand how that set of elements will react in terms of electron gain or loss. However, there are sets of elements that are more complex in their behavior. One such group is the transition elements.
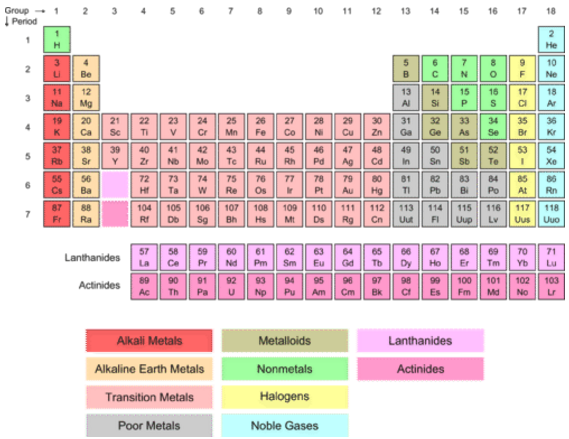
The Transition Elements
Transition elements are the elements that are found in Groups 3-12 (old groups IIA-IIB) on the periodic table (salmon-colored block in the middle of the table). The term refers to the fact that the \(d\) sublevel, which is in the process of being filled, is in a lower principal energy level than the \(s\) sublevel filled before it. For example, the electron configuration of scandium, the first transition element, is \(\left[ \ce{Ar} \right] \: 3d^1 \: 4s^2\). Remember that the configuration is reversed from the fill order—the \(4s\) fills before the \(3d\) begins. Because they are all metals, the transition elements are often called the transition metals. As a group, they display typical metallic properties and are less reactive than the metals in Groups 1 and 2. Some of the more familiar ones are so unreactive that they can be found in nature in their free, or uncombined, state. These include platinum, gold, and silver. Because of this unique filling order, the transition elements are often referred to as "\(d\)-block" elements.

Compounds of many transition elements are distinctive for being widely and vividly colored. As visible light passes through a transition metal compound dissolved in water, the \(d\)-orbitals absorb light of various energies. The visible light of a given energy level that is not absorbed produces a distinctly colored solution.

Summary
- The transition elements are found in groups IIA-IIB (new groups 3-12).
- These elements are characterized by having unfilled \(d\) sublevels.
- In general, the next higher \(s\) sublevel is already filled or has one electron missing.
- Many transition element compounds are brightly colored due to the inner-level \(d\) electron transitions.
Review
- List five different transition elements, giving their name, chemical symbol, and atomic number.
- What is unique about the transition elements in terms of electron configurations?
- Why are these elements often referred to as “\(d\)-block” elements?
- Why do many transition element compounds have bright colors?

