6.14: Lanthanides and Actinides
- Page ID
- 53706
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)How many dolls are in this picture?
Russian "nesting dolls" (often known as matryoshka dolls) have a long history in Russia. These dolls are designed to nest inside of one another. When we open the largest doll, we find a slightly smaller doll inside it. These dolls can often go down seven or eight layers, and some have over thirty-five layers.
Lanthanides and Actinides
We see some hidden "layers" in chemistry. Examining the periodic table below, visible are two pink boxes—one between \(\ce{Ba}\) (element 56) and \(\ce{Hf}\) (element 72) and the other between \(\ce{Ra}\) (88) and \(\ce{Rf}\) (104). These elements all have unfilled \(f\)-sublevels. Because of the uniqueness of the electron configurations, these elements fit into the two boxes in the larger periodic table.
As the number of electrons in an atom increases, we begin to see some strange behaviors. Due to the way the electron energy levels work, some inner levels fill after one or more outer layers do. We see this in two similar groups of elements—the lanthanides and the actinides.
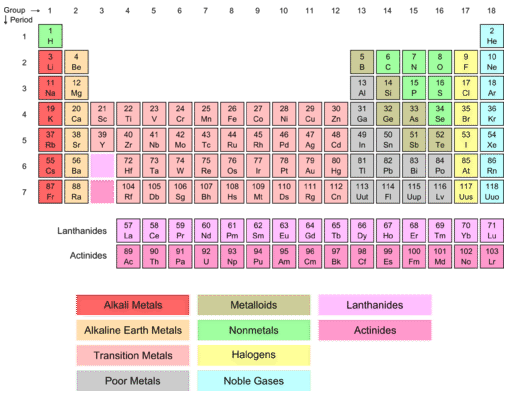
The \(f\)-Block
The first of the \(f\) sublevels to begin filling is the \(4f\) sublevel. It fills after the \(6s\) sublevel, meaning that \(f\) sublevels are two principal energy levels behind. The general electron configuration for elements in the \(f\)-block is \(\left( n-2 \right) f^{1-14} ns^2\). The seven orbitals of the \(f\) sublevel accommodate 14 electrons, so the \(f\) block is 14 elements in length. It is pulled out of the main body of the periodic table and is shown at the very bottom. Because of that, the elements of the \(f\) block do not belong to a group, being wedged in between Groups 3 and 4. The lanthanides are the 14 elements from cerium (atomic number 58) to lutetium (atomic number 71). The word comes from the Greek "\(\lambda \alpha \nu \theta \alpha \nu \epsilon \iota \nu \)" meaning "to be hidden". The name probably arose because these elements all hide behind one another in the periodic table. The \(4f\) sublevel is in the process of being filled for the lanthanides. They are all metals and are similar in reactivity to the Group 2 alkaline earth metals.
The actinides are the 14 elements from thorium (atomic number 90) to lawrencium (atomic number 103). The \(5f\) sublevel is in the process of being filled. The actinides are all radioactive elements and only the first four have been found naturally on Earth. All of the others have only been artificially made in the laboratory. The lanthanides and actinides together are sometimes called the inner transition elements.
Uses of Lanthanides
Lanthanides have been widely used as alloys to impart strength and hardness to metals. The main lanthanide used for this purpose is cerium, mixed with small amounts of lanthanum, neodymium, and praseodymium. These metals are also widely used in the petroleum industry for refining crude oil into gasoline products.

Erbium and other lanthanides are widely used in some optical devices, such as night vision goggles, laser beams, and phosphorescent materials.
Uses of Actinides
The actinides are valuable primarily because they are radioactive. These elements can be used as energy sources for applications as varied as cardiac pacemakers, to the generation of electrical energy for instruments on the moon. Uranium and plutonium have been employed in nuclear weapons and in nuclear power plants.

Summary
- Lanthanides and actinides are elements with unfilled \(f\) orbitals.
- Lanthanides are all metals with reactivity similar to group 2 elements.
- Actinides are all radioactive elements.
- Lanthanides are used in optical devices (night vision goggles), petroleum refining, and alloys.
- Actinides are found primarily in applications where their radioactivity can be used to power devices such as cardiac pacemakers.
Review
- What electron sublevel is being filled in the lanthanides?
- What electron sublevel is being filled in the actinides?
- What sublevel is filled just prior to the filling of this sublevel?
- Which actinides are found naturally on earth?
- List some uses for lanthanides.
- List some uses for actinides.

