26.4: Stereoisomerism in Organic Compounds
- Page ID
- 24382
In the 1960’s, a drug called thalidomide was widely prescribed in the Western Europe to alleviate morning sickness in pregnant women.
Thalidomide had previously been used in other countries as an antidepressant, and was believed to be safe and effective for both purposes. The drug was not approved for use in the U.S.A. It was not long, however, before doctors realized that something had gone horribly wrong: many babies born to women who had taken thalidomide during pregnancy suffered from severe birth defects.
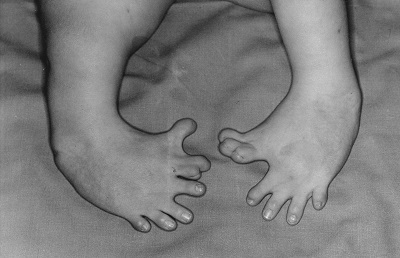
Baby born to a mother who had taken thalidomide while pregnant.
Researchers later realized the that problem lay in the fact that thalidomide was being provided as a mixture of two different isomeric forms.
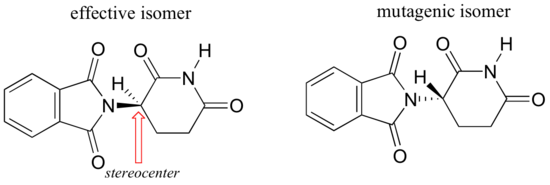
One of the isomers is an effective medication, the other caused the side effects. Both isomeric forms have the same molecular formula and the same atom-to-atom connectivity, so they are not constitutional isomers. Where they differ is in the arrangement in three-dimensional space about one tetrahedral, sp3-hybridized carbon. These two forms of thalidomide are stereoisomers.
Note that the carbon in question has four different substituents (two of these just happen to be connected by a ring structure). Tetrahedral carbons with four different substituent groups are called stereocenters.
Looking at the structures of what we are referring to as the two isomers of thalidomide, you may not be entirely convinced that they are actually two different molecules. In order to convince ourselves that they are indeed different, let’s create a generalized picture of a tetrahedral carbon stereocenter, with the four substituents designated R1-R4. The two stereoisomers of our simplified model look like this:
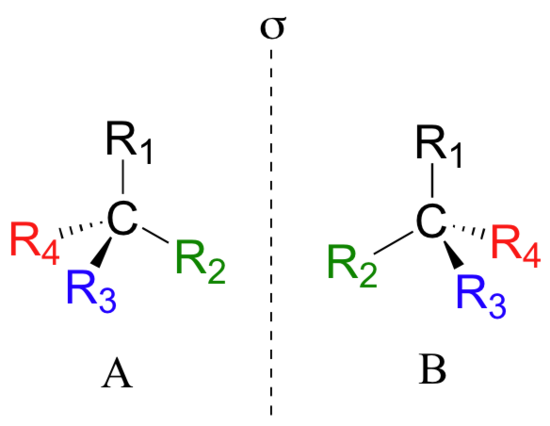
If you look carefully at the figure above, you will notice that molecule A and molecule B are mirror images of each other (the line labeled 's' represents a mirror plane). Furthermore, they are not superimposable: if we pick up molecule A, flip it around, and place it next to molecule B, we see that the two structures cannot be superimposed on each other. They are different molecules!
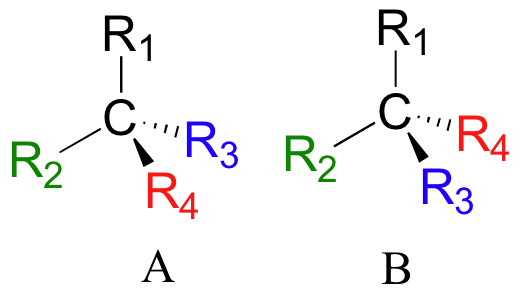
See a figure of the two enantiomers of the amino acid alanine
If you make models of the two stereoisomers of thalidomide and do the same thing, you will see that they too are mirror images, and cannot be superimposed (it well help to look at a color version of the figure below).
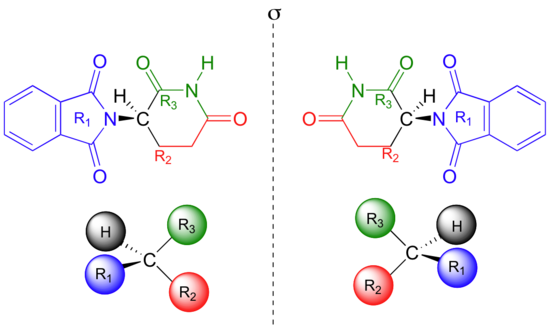
Thalidomide is a chiral molecule. Something is considered to be chiral if it cannot be superimposed on its own mirror image – in other words, if it is asymmetric (lacking in symmetry). The term ‘chiral’ is derived from the Greek word for ‘handedness’ – ie. right-handedness or left-handedness. Your hands are chiral: your right hand is a mirror image of your left hand, but if you place one hand on top of the other, both palms down, you see that they are not superimposable.
A pair of stereoisomers that are non-superimposable mirror images of one another are considered to have a specific type of stereoisomeric relationship – they are a pair of enantiomers. Thalidomide exists as a pair of enantiomers. On the macro level, your left and right hands are also a pair of enantiomers.
Naming Enantiomers: The R,S, System
Chemists need to have a convenient way to distinguish between stereoisomers. The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations ‘R’ (from the Latin rectus, meaning right-handed) or ‘S’ (from the Latin sinister, meaning left-handed).
The rules for this system of stereochemical nomenclature are, on the surface, fairly simple. Because thalidomide is a somewhat complicated molecule, we’ll use the simple 3-carbon sugar glyceraldehyde as our first example. You will want to make a model of the stereoisomer of glyceraldehyde shown below (be sure that you are making the correct enantiomer!).
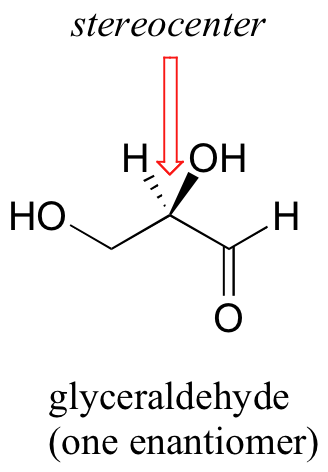
The first thing that we must do is to assign a priority to each of the four substituents bound to the chiral carbon. In this nomenclature system, the priorities are based on atomic number, with higher atomic numbers having a higher priority. We first look at the atoms that are directly bonded to the chiral carbon: these are H, O (in the hydroxyl), C (in the aldehyde), and C (in the CH2OH group).
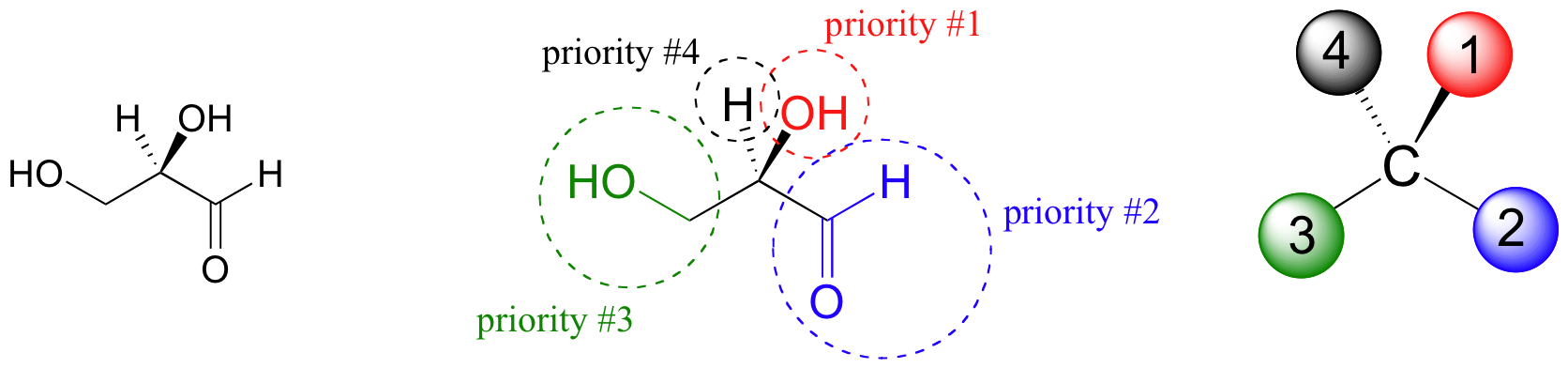
Two priorities are easy: hydrogen, with an atomic number of 1, is the lowest (#4) priority, and the hydroxyl oxygen, with atomic number 8, is priority #1. Carbon has an atomic number of 6. Which of the two ‘C’ groups is priority #2, the aldehyde or the CH2OH? To determine this, we move one more bond away from the stereocenter: for the aldehyde we have a double bond to an oxygen, while on the CH2OH group we have a single bond to an oxygen. If the atom is the same, double bonds have a higher priority than single bonds. Therefore, the aldehyde group is assigned #2 priority and the CH2OH group the #3 priority. With our priorities assigned, we next make sure that the #4 priority group (the hydrogen) is pointed back away from ourselves, into the plane of the page (it is already).

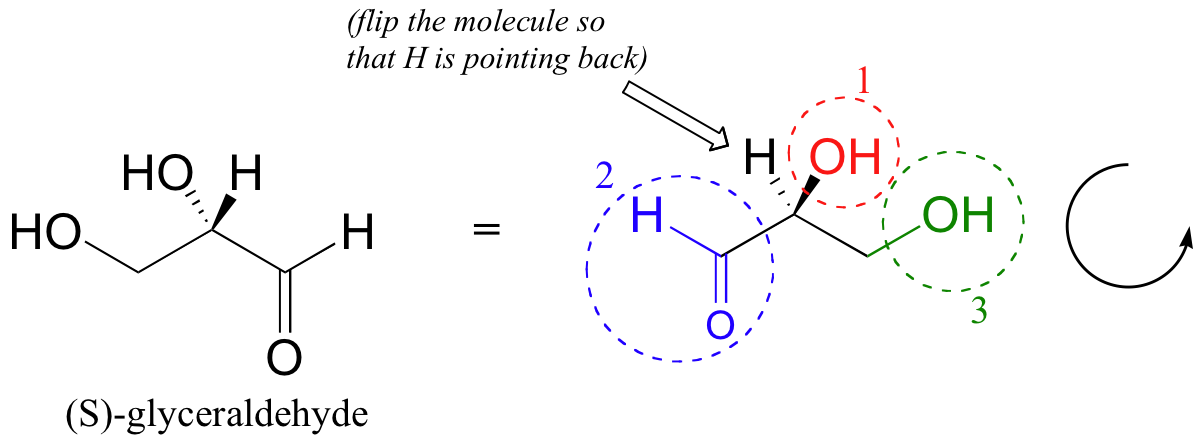
Then, we trace a circle defined by the #1, #2, and #3 priority groups, in increasing order. For our glyceraldehyde example, this circle is clockwise, which tells us that this carbon has the ‘R’ configuration, and that this molecule is (R)-glyceraldehyde. For (S)-glyceraldehyde, the circle described by the #1, #2, and #3 priority groups is counter-clockwise (but first, we must flip the molecule over so that the H is pointing into the plane of the page).
We can follow a similar procedure for a molecule such as lactate. Clearly, H is the #4 substituent and OH is #1. Owing to its three bonds to oxygen, the CO2 group takes priority #2.
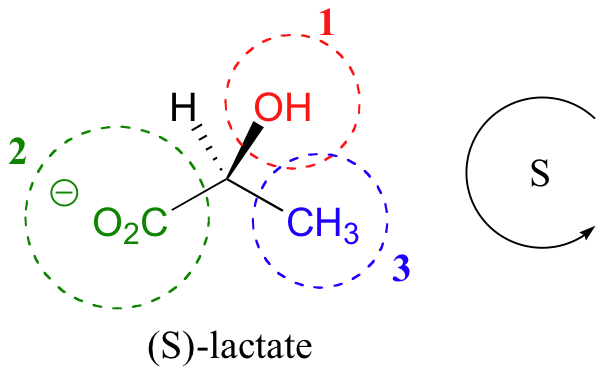
The structure shown is the S enantiomer of lactate.
Now, let’s try to assign a stereochemical configuration to the enantiomer of thalidomide that is the effective medication. The #4 priority is again hydrogen (in fact, the #4 group will be hydrogen for most of the stereocenters we encounter). The nitrogen group is #1, the carbonyl side of the ring is #2, and the –CH2 side of the ring is #3.
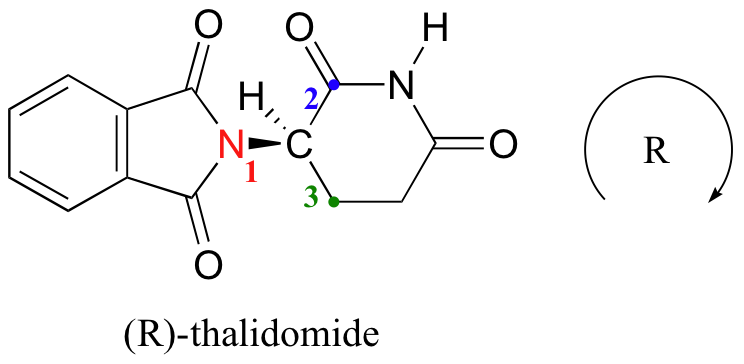
The hydrogen is shown pointing away from us, and the prioritized substituents trace a clockwise circle: this is the R enantiomer of thalidomide.
Label stereochemistry for the figures of glyceraldehyde, alanine, lactate and amphetamine shown earlier in the chapter (section 3.3).
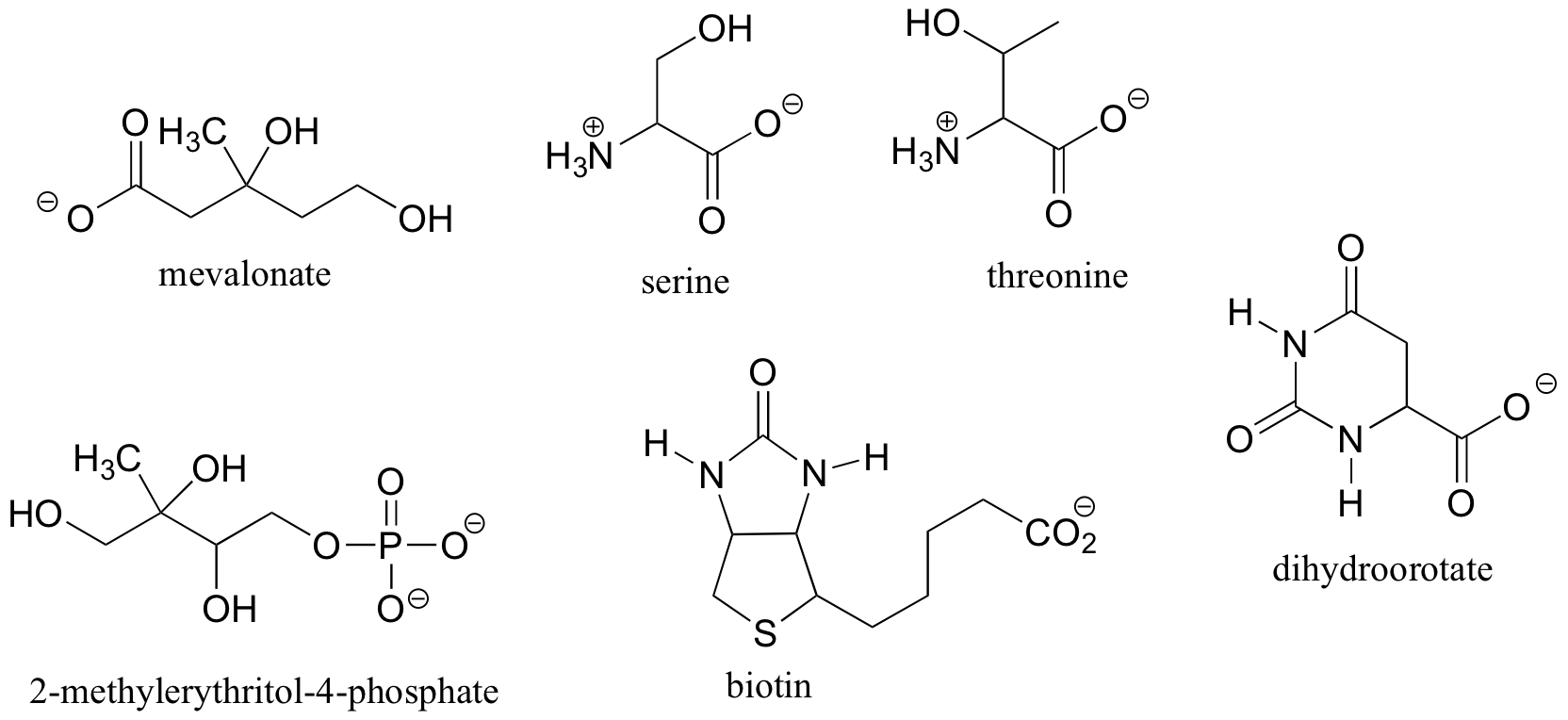
Using solid or dashed wedges to show stereochemistry, draw the (R)-enantiomers of malate and ibuprofin (structures in exercise 3.6, this section), and mevalonate, 2-methylerithritol-4-phosphate, and dihydroorotate (structures in section 3.3).
Summary
Stereoisomers are isomers that differ in spatial arrangement of atoms, rather than order of atomic connectivity. One of their most interesting type of isomer is the mirror-image stereoisomers, a non-superimposable set of two molecules that are mirror image of one another. The existance of these molecules are determined by concept known as chirality. The word "chiral" was derived from the Greek word for hand, because our hands display a good example of chirality since they are non-superimposable mirror images of each other.
Contributors and Attributions
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)