Nutrition
- Page ID
- 461
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The use of food by organisms is termed nutrition. Vitamins and minerals necessary for biochemical processes. There are three general categories of food: (1) Essential fiber which are non-digestible polysaccharide material, essential for normal functioning of animal digestive systems (i.e. colon), (2) Energy-yielding nutrients which are protein, carbohydrate and lipid and (3) Micronutrients.
Protein
Animals are unable to synthesize certain amino acids (humans can only make 10 of the 20 common amino acids). The amino acids that an animal is unable to synthesize must be obtained from the diet (i.e. by consuming plants or microorganisms), and these amino acids are termed "essential amino acids".
Excess dietary protein becomes a source of metabolic energy
- Glucogenic amino acids: can be converted into glucose
- Ketogenic amino acids: can be converted into fatty acids or keto acids
- If plenty of fats and carbohydrates are available, then the glucogenic and ketogenic amino acids from excess dietary protein are converted to triacylglycerol and stored as fat
Protein is an important source of nitrogen in the diet. Protein within the body is constantly turning over (i.e. being degraded and resynthesized). Furthermore, there is a general demand for protein synthesis when an organism is growing. The Nitrogen Balance refers to the relationship between the supply and demand for nitrogen (i.e. protein) within an organism.
- A positive nitrogen balance means that the organism is taking in more protein that it needs for growth or turnover
- A negative nitrogen balance means that the organism is not getting enough protein for its normal turnover, or growth. This would represent a nutritional deficiency of protein
Carbohydrate
Carbohydrates are also an essential structural component of nucleic acids, nucleotides, glycoproteins and glycolipids. However, the principle role of carbohydrate in the diet is production of metabolic energy.
- Simple sugars are metabolized in the glycolytic pathway to release energy
- Complex carbohydrates are degraded into simple sugars, which then enter the glycolytic pathway
- Metabolism can make use of a wide variety of sugars for energy production. However, the brain relies solely on glucose for an energy source
- When dietary carbohydrate exceeds the supply needed for energy requirements, it is converted to glycogen and triacylglycerols for storage
- When dietary carbohydrate is in short supply, ketone bodies are formed from acetate units to provide fuel for the brain
Lipids
Fatty acids and triacylglycerols can be used as fuel by many tissues in the human body. Phospholipids are essential components of all biological membranes
- Excess dietary fat is stored as triacylglycerols in adipose tissue
- A deficiency of dietary fat is problematic because some fatty acids cannot be synthesized by the human body, and must be obtained through diet. These are termed
- essential fatty acids
- The human body cannot synthesize linoleic, linolenic or arachidonic fatty acids. These are key components of biological membranes, and arachidonic acid is a precursor of prostaglandins (an important class of hormones). These are therefore considered essential fatty acids
Fiber
"Dietary fiber" refers to molecules that cannot be broken down by enzymes in the human body.
- Cellulose (polysaccharide component of plant cell walls). Required for proper function of colon.
- Lignins (plant polymer of aromatic ring structures). Absorbs organic molecules in the digestive system (binds cholesterol).
Vitamins and minerals
Vitamins are essential nutrients that are required in the diet because they cannot be synthesized by human metabolic enzymes. Often, only trace levels are required, but a shortage can result in disease or death.
- A common categorization of human vitamins is whether they are
- water soluble or fat soluble compounds.
Coenzymes are low molecular weight molecules that provide unique chemical functionalities for certain enzyme/coenzyme complexes.
- Coenzymes may act as carriers of specific functional groups (e.g. methyl or acyl groups)
- They can provide chemically reactive groups that the common 20 amino acid side chains cannot provide
- Coenzymes are
- usually modified in the course of a reaction, and subsequently chemically regenerated back to their useful active form. Thus, this recycling of coenzymes means that only small concentrations are required.
- All the
- water soluble vitamins (with the exception of vitamin C) are coenzymes or precursors of coenzymes.
Summary of water soluble and fat soluble vitamins:
|
Common name |
Chemical name |
Related cofactor(s) |
|
Water Soluble Vitamins |
||
|
Vitamin B1 |
Thiamine |
Thiamine pyrophosphate |
|
Vitamin B2 |
Riboflavin |
Flavin adenine dinucleotide (FAD) |
|
Vitamin B6 |
Pyridoxal, pyridoxine, pyridoxamine |
Pyridoxal phosphate |
|
Vitamin B12 |
Cobalamin |
5'-deoxyadenosylcobalamin |
|
Niacin |
Nicotinic acid |
Nicotinamide adenine dinucleotide (NAD+) |
|
Vitamin B3 |
Pantothenic acid |
Coenzyme A |
|
Biotin |
Biotin-lysine conjugates (biocytin) |
|
|
Lipoic acid |
Lipoyl-lysine conjugates (lipoamide) |
|
|
Folic acid |
Tetrahydrofolate |
|
|
Vitamin C |
L-ascorbate |
|
|
Fat Soluble Vitamins |
||
|
Vitamin A |
Retinol |
|
|
Vitamin D2 |
Ergocalciferol |
|
|
Vitamin D3 |
Cholecalciferol |
|
|
Vitamin E |
a-Tocopherol |
|
|
Vitamin K |
||
Vitamin B1: Thiamine and thiamine pyrophosphate
Thiamine is the precursor of thiamine pyrophosphate (TPP):
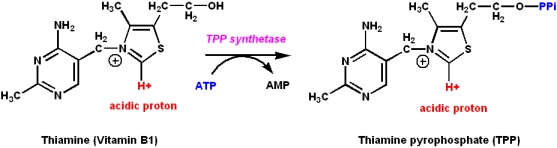
- TPP is a coenzyme
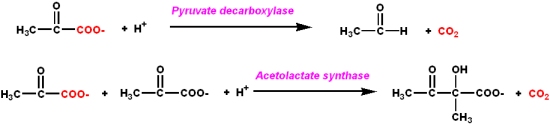
- It is a coenzyme for certain enzymes involved in carbohydrate metabolism
- It catalyzes the synthesis or cleavage of bonds to carbonyl carbons
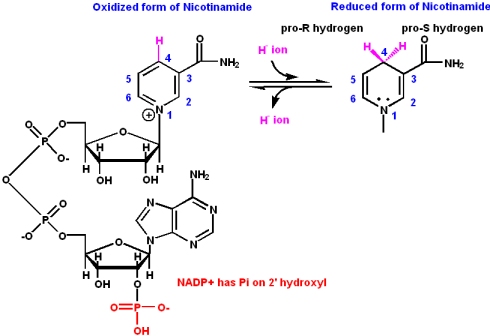
Niacin (Nicotinic Acid) and nicotinamide coenzymes
Nicotinamide is an essential part of two important coenzymes: nicotinamide adenine dinucleotide (NAD+) and nicotinamide adenine dinucleotide phosphate (NADP+).
- The reduced forms of these coenzymes are NADH and NADPH
- The coenzymes participate in
- redox reactions via the direct transfer of hydride (H-) ions either to or from the cofactor and a substrate. The hydride transfer carries two electrons along with it (a proton transfer in acid/base catalysis carries no electrons)
- The hydride transfer involves the C4 carbon of the nicotinamide ring. The quaternary amine of the nicotinamide ring acts as an electron sink to promote acceptance of a hydride ion, or to facilitate leaving of a hydride ion.
- Enzymes that are involved in such redox reactions are called
- dehydrogenases
- The nucleotide part of the molecule does not enter into any chemistry, but
- is important for recognition and binding to enzymes that will use FMN or FAD as a cofactor.
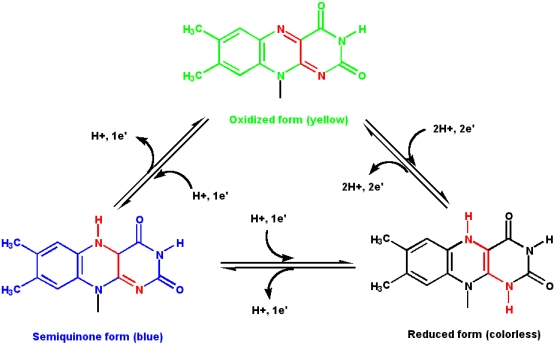
Vitamin B2: Riboflavin
Riboflavin is a constituent of riboflavin 5'-phosphate (flavin mononucleotide, or FMN) and flavin adenine dinucleotide (FAD). The nucleotide part of the molecule does not enter into any chemistry, but is important for recognition and binding to enzymes that will use FMN or FAD as a cofactor.
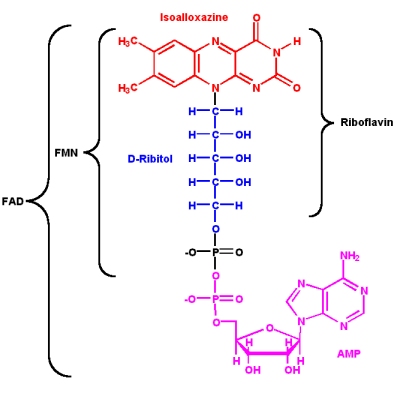
The isoalloxazine ring is the core structure of the different flavin molecules. It is yellow in color and the word "flavin" is derived from the latin word for yellow, flavus.
- Flavin coenzymes can exists in three different redox states, and each state has a different color (the reduced form is colorless)
- Flavin molecules can participate in both
- one- and two- electron transfer reactions
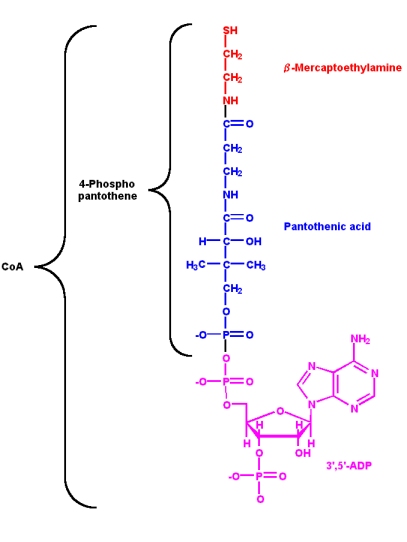
Vitamin B3: pantothenic acid and coenzyme A
Pantothenic acid is a component of coenzyme A (CoA). The two main functions of CoA are:
a-hydrogen of the acyl group for removal as a proton
- Activation of acyl groups (R-COX) for transfer to nucleophilic acceptors
- Activation of the
Both of these functions involve the reactive sulfhydryl group through the formation of thioester linkages with acyl groups
- The 4-phosphopantetheine part of CoA is also used in the same way in
- acyl carrier proteins (ACP's) involved in fatty acid biosynthesis
Vitamin B6: Pyridoxine and pyridoxal phosphate
The biologically active form of vitamin B6 is pyridoxal-5-phosphate (PLP), however, the nutritional requirements can be met by either pyridoxine, pyridoxal or pyridoxol.
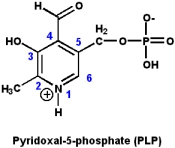
PLP participates in a wide variety of reactions involving amino acids, including:
- Transamination
- a- and b-decarboxylation
- b- and g- elimination (not to be confused with painful elimination)
- Racemization
- Aldol reactions
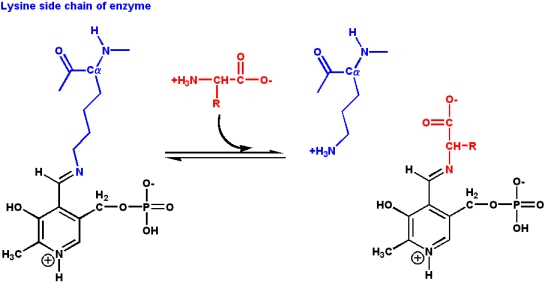
These involve bonds to the amino acid Ca as well as side chain carbons. The wide variety of reactions is due to the ability of PLP to form stable Schiff base adducts with a-amino groups of amino acids:
- In PLP-dependent enzymes, the PLP is present in a Schiff base linkage with the
- e-amino group of an acitve site lysine
- Rearrangement to a Schiff base with the arriving amino acid substrate is a
- transaldiminization reaction
Vitamin B12: Cyanocobalamin
Vitamin B12 is not made by any animal or plant, it is produced by only a few species of bacteria. Once in the food chain, vitamin B12 is obtained by animals by eating other animals, but plants are sadly deficient. Therefore, herbivorous animals (and vegetarians) can suffer a deficiency. The structure contains a cobalt ion, coordinated within a corrin ring structure:
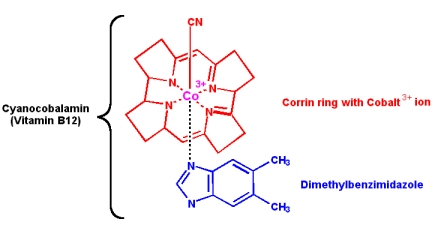
Vitamin B12 (cyanocobalamin) is converted in the body into two coenzymes:
- 5'-deoxyadenosylcobalamin (the predominant form)
- Methylcobalamin
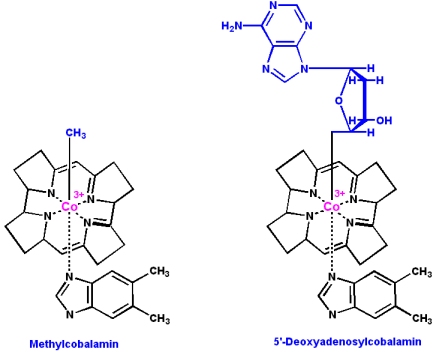
Vitamin B12 coenzymes participate in three types of reactions:
- Intramolecular rearrangements
- Reductions of ribonucleotides to deoxyribonucleotides in certain bacteria
- Methyl group transfers (these use methylcobalamin for this purpose)
Vitamin C (L-Ascorbate)
L-Ascorbate is a reducing sugar (has a reactive ene-diol structure) that is involved in the following biochemical processes:
- Hydroxylation of proline and lysine residues in collagen. Without these post-translational modifications the triple helix of collagen is unstable and connective tissue loses its integrity. This is the problem in the disease known as
- scurvy.
- Mobilization of iron, stimulation of immune system, anti-oxidant for scavenging of reactive free-radicals.
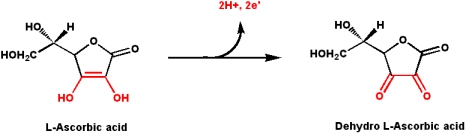
Almost all animals can synthesize vitamin C (its in the pathway of carbohydrate synthesis). Humans and great apes have suffered a mutation in the last enzyme in the pathway of synthesis for L-ascorbate (mutation occurred about 10-40 million years ago). Since that time, all great apes (of which humans are a member) must get L-ascorbate from their diet (fresh fruits and vegetable contain an abundance). Thus, for the great apes L-ascorbate is a "vitamin" (another way of looking at it is that all great apes suffer an in-born error in metabolism). Humans still have the gene for the enzyme to make vitamin C. However, it has suffered a couple of deletions that introduce a frame shift mutation, in addition to numerous point mutations.
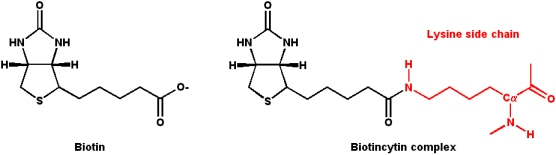
Biotin
Biotin acts as a mobile carboxyl group carrier in a variety of enzymatic carboxylation reactions.
- Synthesized by intestinal bacteria (finaly, they do something for you)
- Biotin is bound covalently to the enzyme as a prosthetic group via an
- e-amino group of a lysine residue in the enzyme
- This biotin-lysine conjugated amino acid is termed a "biocytin" residue
- The lysine side-chain acts as a flexible "tether" for the biotin, and this flexibility allows the transfer of carboxylate groups within the enzyme
- It is the carrier for the most oxidized form of carbon
- - CO2 (using bicarbonate as the carboxylating agent). The carbon dioxide binds as a carboxy group to one of the ring nitrogens in the biotin
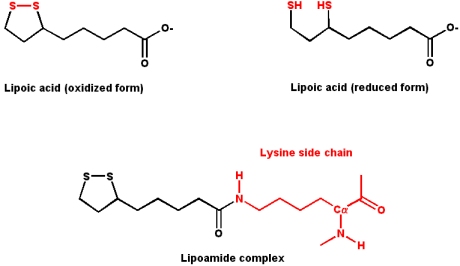
Lipoic Acid
Lipoic acid contains two sulfur atoms that can exist as a disulfide bonded pair, or as two free sulfhydrils. Conversion between the two forms involves a redox reaction (the two free sulfhydrils represent the reduced form). Lipoic acid is typically found covalently attached to a lysine side chain in enzymes that use it as a cofactor, as a lipoamide complex.
- Lipoic acid is an
- acyl group carrier (R-CO-X)
- It also functions to transfer electrons during oxidation and decarboxylation of
- a-keto acids
- Pyruvate dehydrogenase and
- a-ketoglutarate dehydrogenase use lipoic acid as a cofactor
- Its not clear whether a dietary deficiency of lipoic acid contributes to a disease state, so its not technically considered a vitamin
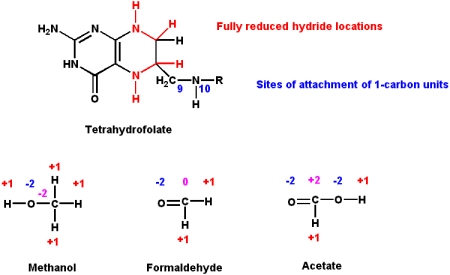
Folic Acid
Folic acid derivatives (i.e. "folates") are acceptors and donors of one-carbon units for all oxidation levels of carbon (except for the most oxidized form - CO2. See biotin above).
- The active coenzyme form is tetrahydrofolate (THF). Folate is reduced to THF by the action of tetrahydrofolate reductase.
- Three different oxidation states of carbon can bind to THF. These are oxidation states of -2 (methanol group), 0 (formaldehyde group) and +2 (formate group)
- These groups are attached to the THF molecule at either the N5 or N10 atom positions
- The biosynthetic pathways of
- methionine, homocysteine, purines, and thymine rely one one-carbon units being provided by THF.
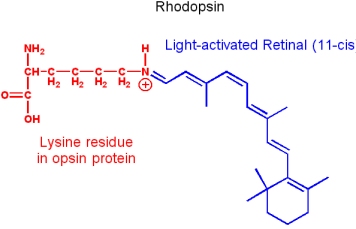
Vitamin A: Retinol
Vitamin A occurs as an ester (Retinyl ester), aldehyde (Retinal) or acidic form (Retinoic acid).
- It is a fat soluble vitamin, and is synthesized from isoprene building blocks
- Obtained directly from an animal diet, or synthesized from
- b-carotene provided by plants
- It is essential to vision. Retinol transported to the eyes is oxidized by retinol dehydrogenase to produce trans-retinal. Trans-retinal is converted to 11-cis-retinal by retinal isomerase. The aldehyde group of retinal forms a Schiff base with a lysin of the protein opsin, to form rhodopsin (the light-sensitive pigment of vision).
- Vitamin A is essential for various biological processes - including fetal development and sperm development. But excessive vitamin A is toxic.
Vitamin D: Ergocalciferol (D2) and cholecalciferol (D3)
Cholecalciferol is produced in the skin of animals by the action of U.V. light on the precursor molecule 7-dehydrocholesterol.
- Light energy induces bond-breakage (between carbons 9 and 10) and formation of previtamin D3. Spontaneous isomerization produces D3
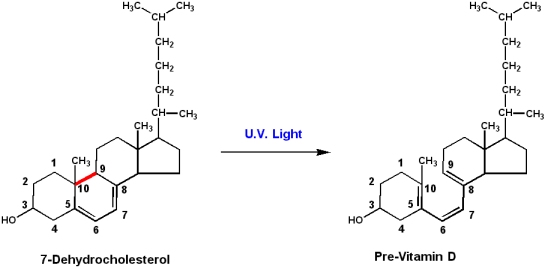
- Ergocalciferol is produced by the action of U.V. light on the plant sterol ergosterol.
- Since humans can produce D3 from 7-dehydrocholesterol, vitamin D3 is technically not really a vitamin
- Cholecalciferol is really a prohormone. Derivatives of this compound
- regulate calcium and phosphate metabolism.
- Inadequate intestinal absorption of calcium and phosphate can result in demineralization of bones, and the disease Rickets.
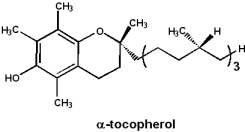
Vitamin E: Tocopherol
a-Tocopherol is a potent antioxidant, however, molecular details of its function are not clearly understood.
- Fatty acids in membranes are susceptible to oxidative damage
- Vitamin E is fat soluble and may protect membrane fatty acids from oxidation
- A deficiency of vitamin E results in red blood cells that are susceptible to oxidative damage
- Retinal damage in premature infants, due to supplemental oxygen, may be preventable by administering vitamin E
Vitamin K: Napthoquinone
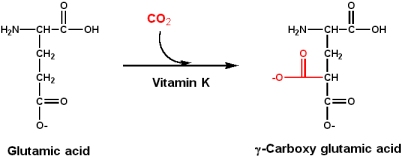
Vitamin K is essential to the blood-clotting process. Vitamin K is required for the post-translational modification to produce g-carboxy glutamic acid from glutamic acid. Such modified residues can bind Ca2+, which is an essential part of the process in the clotting cascade.
g-carboxy glutamic acids in their structure
- Prothrombin ("factor II"), and factors VII, IX and X are serine proteases that participate in a protease activation cascade that is involved in blood coagulation, and have
Outside Links
Contributors and Attributions
- Dr Michael Blaber

