4.6: Looking for Patterns- The Periodic Law and the Periodic Table
- Page ID
- 48581
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how elements are organized into the periodic table.
- Describe how some characteristics of elements relate to their positions on the periodic table.
In the 19th century, many previously unknown elements were discovered, and scientists noted that certain sets of elements had similar chemical properties. For example, chlorine, bromine, and iodine react with other elements (such as sodium) to make similar compounds. Likewise, lithium, sodium, and potassium react with other elements (such as oxygen) to make similar compounds. Why is this so?
In 1864, Julius Lothar Meyer, a German chemist, organized the elements by atomic mass and grouped them according to their chemical properties. Later that decade, Dmitri Mendeleev, a Russian chemist, organized all the known elements according to similar properties. He left gaps in his table for what he thought were undiscovered elements, and he made some bold predictions regarding the properties of those undiscovered elements. When elements were later discovered whose properties closely matched Mendeleev’s predictions, his version of the table gained favor in the scientific community. Because certain properties of the elements repeat on a regular basis throughout the table (that is, they are periodic), it became known as the periodic table.
Mendeleev had to list some elements out of the order of their atomic masses to group them with other elements that had similar properties.
The periodic table is one of the cornerstones of chemistry because it organizes all of the known elements on the basis of their chemical properties. A modern version is shown in Figure \(\PageIndex{1}\). Most periodic tables provide additional data (such as atomic mass) in a box that contains each element’s symbol. The elements are listed in order of atomic number.
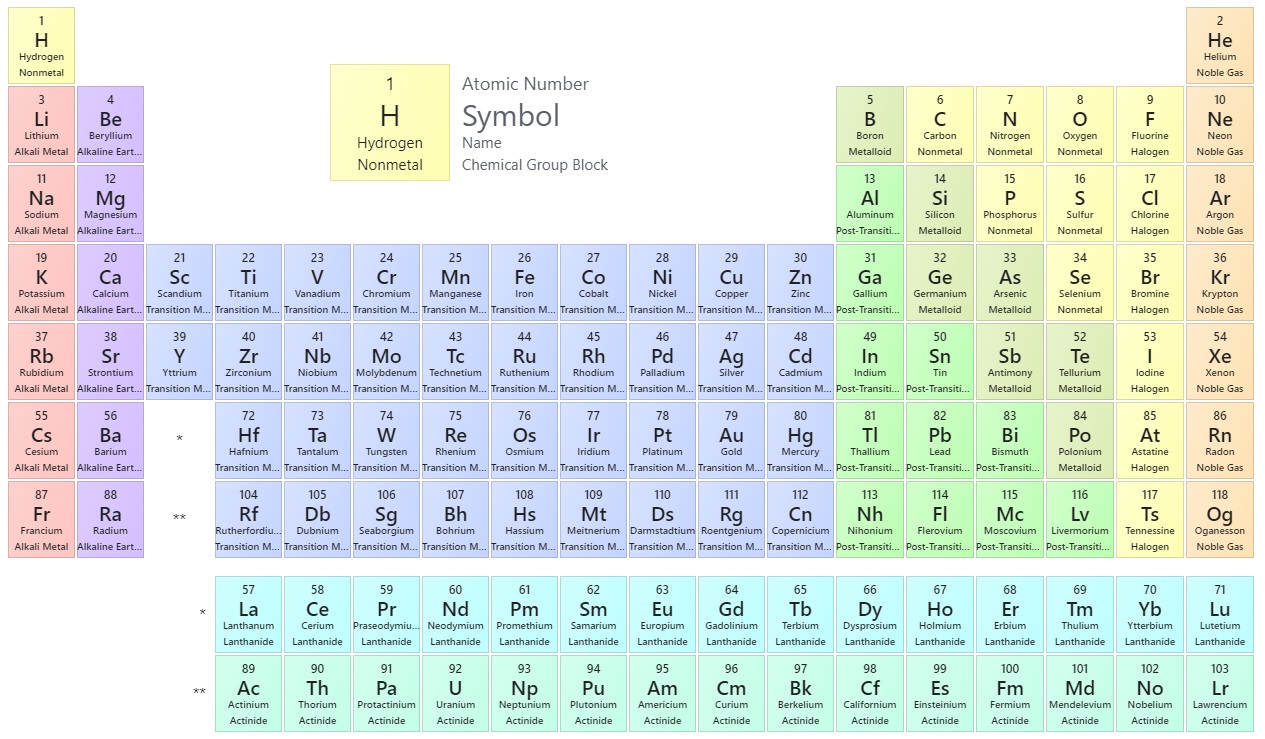
Features of the Periodic Table
Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
The word halogen comes from the Greek for “salt maker” because these elements combine with other elements to form a group of compounds called salts.
Radon is an invisible, odorless noble gas that is slowly released from the ground, particularly from rocks and soils whose uranium content is high. Because it is a noble gas, radon is not chemically reactive. Unfortunately, it is radioactive, and increased exposure to it has been correlated with an increased lung cancer risk.
Because radon comes from the ground, we cannot avoid it entirely. Moreover, because it is denser than air, radon tends to accumulate in basements, which if improperly ventilated can be hazardous to a building’s inhabitants. Fortunately, specialized ventilation minimizes the amount of radon that might collect. Special fan-and-vent systems are available that draw air from below the basement floor, before it can enter the living space, and vent it above the roof of a house.
After smoking, radon is thought to be the second-biggest preventable cause of lung cancer in the United States. The American Cancer Society estimates that 10% of all lung cancers are related to radon exposure. There is uncertainty regarding what levels of exposure cause cancer, as well as what the exact causal agent might be (either radon or one of its breakdown products, many of which are also radioactive and, unlike radon, not gases). The US Environmental Protection Agency recommends testing every floor below the third floor for radon levels to guard against long-term health effects.
Each row of elements on the periodic table is called a period. Periods have different lengths; the first period has only 2 elements (hydrogen and helium), while the second and third periods have 8 elements each. The fourth and fifth periods have 18 elements each, and later periods are so long that a segment from each is removed and placed beneath the main body of the table.
Certain elemental properties become apparent in a survey of the periodic table as a whole. Every element can be classified as either a metal, a nonmetal, or a metalloid (or semi metal), as shown in Figure \(\PageIndex{2}\). A metal is a substance that is shiny, typically (but not always) silvery in color, and an excellent conductor of electricity and heat. Metals are also malleable (they can be beaten into thin sheets) and ductile (they can be drawn into thin wires). A nonmetal is typically dull and a poor conductor of electricity and heat. Solid nonmetals are also very brittle. As shown in Figure \(\PageIndex{2}\), metals occupy the left three-fourths of the periodic table, while nonmetals (except for hydrogen) are clustered in the upper right-hand corner of the periodic table. The elements with properties intermediate between those of metals and nonmetals are called metalloids (or semi-metals). Elements adjacent to the bold line in the right-hand portion of the periodic table have semimetal properties.
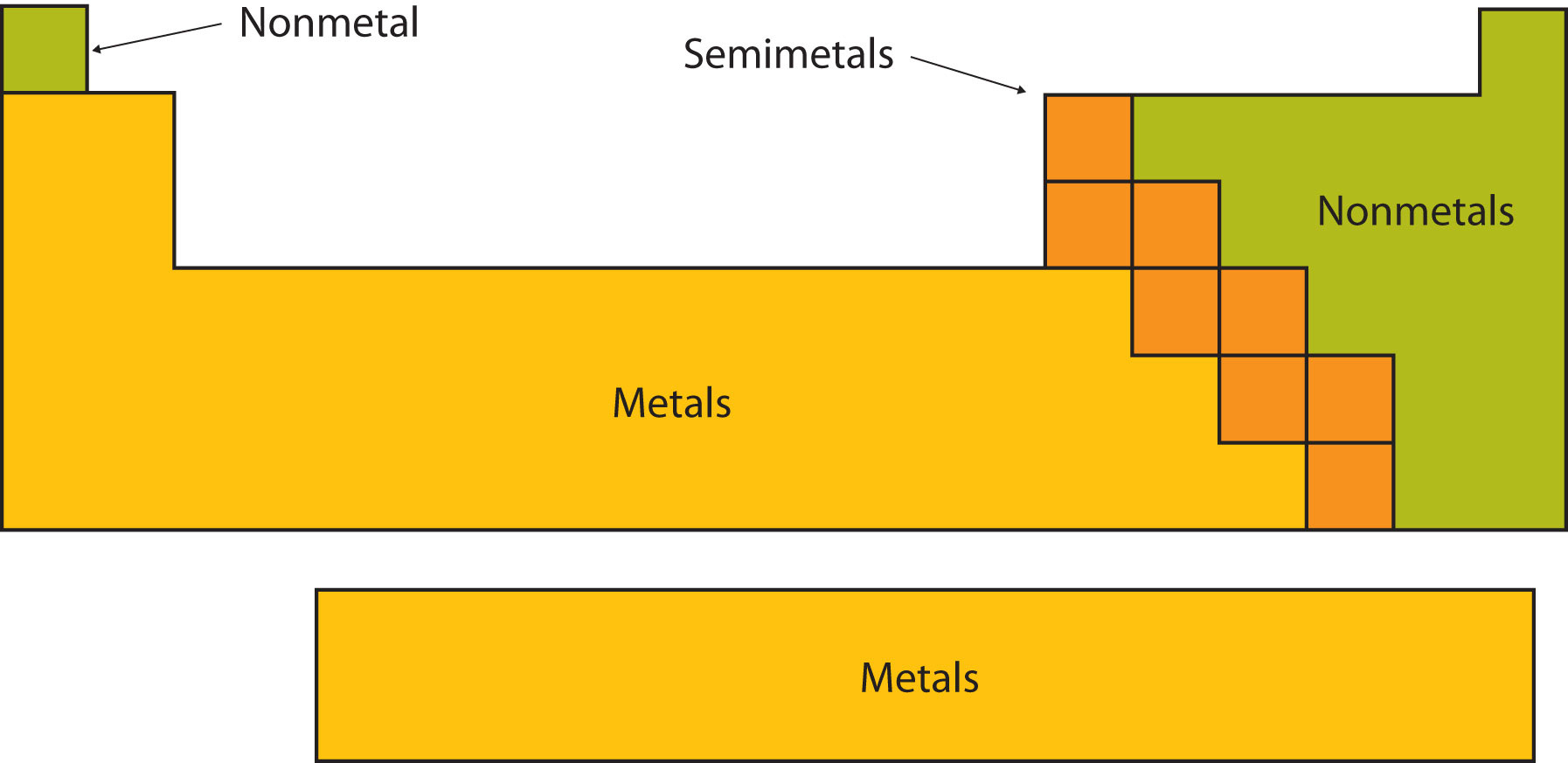
Based on its position in the periodic table, classify each element below as metal, a nonmetal, or a metalloid.
- Se
- Mg
- Ge
Solution
- In Figure \(\PageIndex{1}\), selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so it should be a nonmetal.
- Magnesium lies to the left of the diagonal line marking the boundary between metals and nonmetals, so it should be a metal.
- Germanium lies within the diagonal line marking the boundary between metals and nonmetals, so it should be a metalloid.
Based on its location in the periodic table, do you expect indium to be a nonmetal, a metal, or a metalloid?
- Answer
- Indium is a metal.
Another way to categorize the elements of the periodic table is shown in Figure \(\PageIndex{3}\). The first two columns on the left and the last six columns on the right are called the main group elements. The ten-column block between these columns contains the transition metals. The two rows beneath the main body of the periodic table contain the inner transition metals. The elements in these two rows are also referred to as, respectively, the lanthanide metals and the actinide metals.
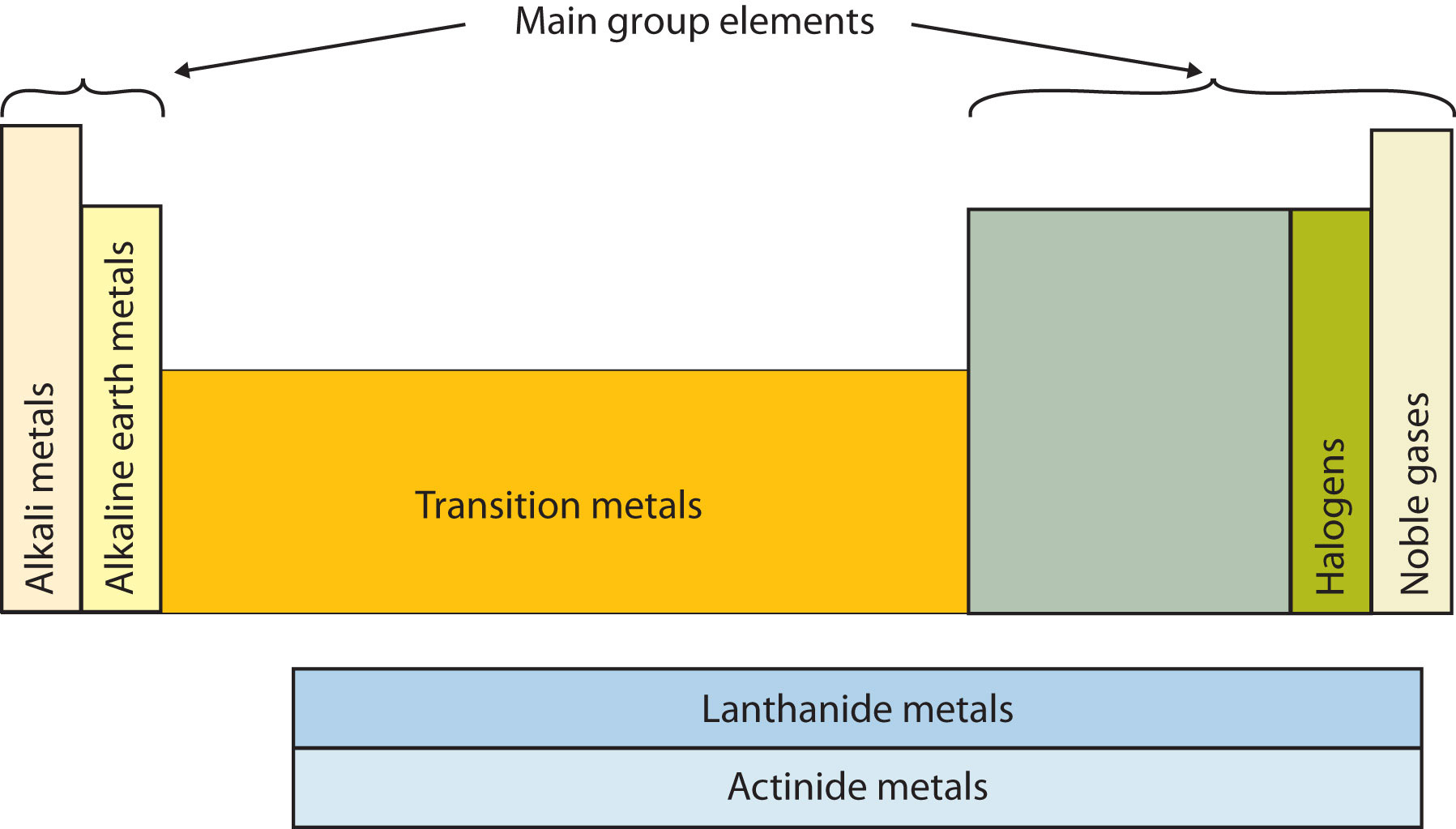
Descriptive Names
As previously noted, the periodic table is arranged so that elements with similar chemical behaviors are in the same group. Chemists often make general statements about the properties of the elements in a group using descriptive names with historical origins.
Group 1: The Alkali Metals
The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is unique in that it is generally placed in Group 1, but it is not a metal.
The compounds of the alkali metals are common in nature and daily life. One example is table salt (sodium chloride); lithium compounds are used in greases, in batteries, and as drugs to treat patients who exhibit manic-depressive, or bipolar, behavior. Although lithium, rubidium, and cesium are relatively rare in nature, and francium is so unstable and highly radioactive that it exists in only trace amounts, sodium and potassium are the seventh and eighth most abundant elements in Earth’s crust, respectively.
Group 2: The Alkaline Earth Metals
The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rare, and radium is unstable and highly radioactive. In contrast, calcium and magnesium are the fifth and sixth most abundant elements on Earth, respectively; they are found in huge deposits of limestone and other minerals.
Group 17: The Halogens
The halogens are fluorine, chlorine, bromine, iodine, and astatine. The name halogen is derived from the Greek words for “salt forming,” which reflects that all of the halogens react readily with metals to form compounds, such as sodium chloride and calcium chloride (used in some areas as road salt).
Compounds that contain the fluoride ion are added to toothpaste and the water supply to prevent dental cavities. Fluorine is also found in Teflon coatings on kitchen utensils. Although chlorofluorocarbon propellants and refrigerants are believed to lead to the depletion of Earth’s ozone layer and contain both fluorine and chlorine, the latter is responsible for the adverse effect on the ozone layer. Bromine and iodine are less abundant than chlorine, and astatine is so radioactive that it exists in only negligible amounts in nature.
Group 18: The Noble Gases
The noble gases are helium, neon, argon, krypton, xenon, and radon. Because the noble gases are composed of only single atoms, they are called monatomic. At room temperature and pressure, they are unreactive gases. Because of their lack of reactivity, for many years they were called inert gases or rare gases. However, the first chemical compounds containing the noble gases were prepared in 1962. Although the noble gases are relatively minor constituents of the atmosphere, natural gas contains substantial amounts of helium. Because of its low reactivity, argon is often used as an unreactive (inert) atmosphere for welding and in light bulbs. The red light emitted by neon in a gas discharge tube is used in neon lights.
Provide the family or group name of each element.
- Li
- Ar
- Cl
Solution
- Lithium is an alkali metal (Group 1)
- Argon is a noble gas (Group 18)
- Chlorine is a halogen (Group 17)
Classify each element as metal, non metal, transition metal or inner transition metal.
- Li
- Ar
- Am
- Fe
Summary
The periodic table is an arrangement of the elements in order of increasing atomic number. Elements that exhibit similar chemistry appear in vertical columns called groups (numbered 1–18 from left to right); the seven horizontal rows are called periods. Some of the groups have widely-used common names, including the alkali metals (Group 1) and the alkaline earth metals (Group 2) on the far left, and the halogens (Group 17) and the noble gases (Group 18) on the far right.
The elements can be broadly divided into metals, nonmetals, and semi metals. Semi metals exhibit properties intermediate between those of metals and nonmetals. Metals are located on the left of the periodic table, and nonmetals are located on the upper right. They are separated by a diagonal band of semi metals. Metals are lustrous, good conductors of electricity, and readily shaped (they are ductile and malleable). Solid nonmetals are generally brittle and poor electrical conductors. Other important groupings of elements in the periodic table are the main group elements, the transition metals, and the inner transition metals (the lanthanides, and the actinides).
References
- Petrucci, Ralph H., William S. Harwood, F. G. Herring, and Jeffrey D. Madura. General Chemistry: Principles and Modern Applications. 9th ed. Upper Saddle River: Pearson Education, Inc., 2007.
- Sisler, Harry H. Electronic structure, properties, and the periodic law. New york; Reinhold publishing corporation, 1963.
- Petrucci, Ralph H., Carey Bissonnette, F. G. Herring, and Jeffrey D. Madura. General Chemistry: Principles and Modern Applications. Custom Edition for CHEM 2. Pearson Learning Solutions, 2010.

